Systematic review of the effect of pneumococcal conjugate vaccine dosing schedules on vaccine-type invasive pneumococcal disease among young children
- PMID: 24336053
- PMCID: PMC3944481
- DOI: 10.1097/INF.0000000000000078
Systematic review of the effect of pneumococcal conjugate vaccine dosing schedules on vaccine-type invasive pneumococcal disease among young children
Abstract
Background: Pneumococcal conjugate vaccines (PCV) are being implemented globally using a variety of different schedules. The optimal schedule to maximize protection of vaccinated children against vaccine-type invasive pneumococcal disease (VT-IPD) is not known.
Methods: To assess the relative benefit of various PCV dosing schedules, we conducted a systematic review of studies published in English from 1994 to 2010 (supplemented post hoc with studies from 2011) on PCV effectiveness against VT-IPD among children targeted to receive vaccine. Data on 2-dose and 3-dose primary series, both with and without a booster ("2+0," "2+1," "3+0" and "3+1"), were included. For observational studies using surveillance data or case counts, we calculated percentage reduction in VT-IPD before and after PCV introduction.
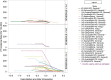
Results: Of 4 randomized controlled trials and 31 observational studies reporting VT-IPD among young children, none evaluated a 2+0 complete series, 7 (19%) evaluated 2+1, 4 (11%) 3+0 and 27 (75%) 3+1. Most (86%) studies were from North America or Europe. Only 1 study (observational) directly compared 2 schedules (3+0 vs. 3+1); results supported the use of a booster dose. In clinical trials, vaccine efficacy ranged from 65% to 71% with 3+0 and 83% to 94% with 3+1. Surveillance data and case counts demonstrate reductions in VT-IPD of up to 100% with 2+1 (6 studies) or 3+1 (17 studies) schedules and up to 90% with 3+0 (2 studies). Reductions were observed as early as 1 year after PCV introduction.
Conclusions: These data support the use of 2+1, 3+0 and 3+1 schedules, although most data of PCV impact on VT-IPD among young children are from high-income countries using 3+1. Differences between schedules for impact on VT-IPD are difficult to discern based on available data.
Conflict of interest statement
Support for this project was provided by Program for Appropriate Technology in Health (PATH) through funding from the GAVI Alliance. The views expressed by the authors do not necessarily reflect the views of CDC, GAVI, PATH or IVAC. M.D.K. has received support from Novartis for participation on a Data and Safety Monitoring Board and meeting travel reimbursement from Pfizer. D.G.’s laboratory performs contract and or collaborative research for/with Pfizer, GlaxoSmithKline, Merck, Novartis and Sanofi Pasteur. D.G. has received travel or honorarium support for participation in external expert committees for Merck, Sanofi Pasteur, Pfizer and GlaxoSmithKline. K.O.B. received grant support from Pfizer, GlaxoSmithKline and has received travel or honorarium support for participation in external expert committees for Merck, Aventis-pasteur and GlaxoSmithKline. The authors have no other funding or conflicts of interest to declare.
Figures

References
-
- van Hoek AJ, Andrews N, Waight PA, et al. The effect of underlying clinical conditions on the risk of developing invasive pneumococcal disease in England. J Infect. 2012;65:17–24. - PubMed
-
- Battersby AJ, Knox-Macaulay HH, Carrol ED. Susceptibility to invasive bacterial infections in children with sickle cell disease. Pediatr Blood Cancer. 2010;55:401–406. - PubMed
-
- Pilishvili T, Lexau C, Farley MM, et al. Active Bacterial Core Surveillance/Emerging Infections Program Network. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41. - PubMed
-
- Centers for Disease Control and Prevention. Progress in introduction of pneumococcal conjugate vaccine—worldwide, 2000–2008. Morb Mortal Wkly Rep. 2008;57:1148–1151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

