Systematic review of the effect of pneumococcal conjugate vaccine dosing schedules on vaccine-type nasopharyngeal carriage
- PMID: 24336057
- PMCID: PMC3940522
- DOI: 10.1097/INF.0000000000000083
Systematic review of the effect of pneumococcal conjugate vaccine dosing schedules on vaccine-type nasopharyngeal carriage
Abstract
Background: Pneumococcal conjugate vaccines (PCV) reduce nasopharyngeal carriage of vaccine type (VT) pneumococci, an important driver of vaccine programs' overall benefits. The dosing schedule that best reduces carriage is unclear.
Methods: We performed a systematic review of English language publications from 1994 to 2010 (supplemented post hoc with studies from 2011) reporting PCV effects on VT carriage to assess variability in effect by dosing schedule.
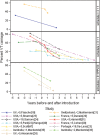
Results: We identified 32 relevant studies (36 citations) from 12,980 citations reviewed. Twenty-one (66%) evaluated PCV7; none used PCV10 or PCV13. Five studies evaluated 2 primary doses and 13 three primary doses. After the first year of life, 14 evaluated 3-dose primary series with PCV booster (3+1), seven 3 doses plus 23-valent polysaccharide booster "3+1PPV23," five "3+0," four "2+1," three "2+1PPV23" and two "2+0." Four studies directly compared schedules. From these, 3 primary doses reduced VT carriage more than 2 doses at 1-7 months following the series (1 study significant; 2 borderline). In a study, the 2+1 schedule reduced VT carriage more than 2+0 at 18, but not at 24 months of age. One study of a 23-valent pneumococcal polysaccharide vaccine booster showed no effect. All 16 clinical trials with unvaccinated controls and 11 observational studies with before-after designs showed reduction in VT carriage.
Conclusions: The available literature demonstrates VT-carriage reduction for 2+0, 2+1, 3+0 and 3+1 PCV schedules, but not for 23-valent pneumococcal polysaccharide vaccine booster. Comparisons between schedules show that 3 primary doses and a 2+1 schedule may reduce carriage more than 2 primary doses and a 2+0 schedule, respectively.
Conflict of interest statement
Support for this project was provided by Program for Appropriate Technology in Health (PATH) through funding from the GAVI Alliance. The views expressed by the authors do not necessarily reflect the views of CDC, GAVI, PATH or IVAC. M.D.K. has received support from Novartis for participation on a Data and Safety Monitoring Board, meeting travel reimbursement from Pfizer and grant support from Merck. D.G.’s laboratory performs contract and or collaborative research for/with Pfizer, GlaxosmithKline, Merck, Novartis and Sanofi Pasteur. D.G. has received travel or honorarium support for participation in external expert committees for Merck, Sanofi Pasteur, Pfizer and GlaxosmithKline. K.O.B. received grant support from Pfizer, GlaxosmithKline and has received travel or honorarium support for participation in external expert committees for Merck, Aventis-pasteur and GlaxosmithKline. The authors have no other funding or conflicts of interest to disclose.
Figures



References
-
- O’Brien KL, Nohynek H World Health Organization Pneumococcal Vaccine Trials Carriage Working Group. Report from a WHO Working Group: standard method for detecting upper respiratory carriage of Streptococcus pneumoniae. Pediatr Infect Dis J. 2003;22:e1–11. - PubMed
-
- Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4:144–154. - PubMed
-
- Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). Morb Mort Wkly Rep. 2010;59:1102–1106. - PubMed
-
- Nuorti JP, Whitney CG Centers for Disease Control and Prevention (CDC) Prevention of pneumococcal disease among infants and children—use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010;59(RR-11):1–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

