Systematic review of the indirect effect of pneumococcal conjugate vaccine dosing schedules on pneumococcal disease and colonization
- PMID: 24336058
- PMCID: PMC3940524
- DOI: 10.1097/INF.0000000000000084
Systematic review of the indirect effect of pneumococcal conjugate vaccine dosing schedules on pneumococcal disease and colonization
Abstract
Background: To aid decision making for pneumococcal conjugate vaccine (PCV) use in infant national immunization programs, we summarized the indirect effects of PCV on clinical outcomes among nontargeted age groups.
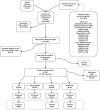
Methods: We systematically reviewed the English literature on infant PCV dosing schedules published from 1994 to 2010 (with ad hoc addition of 2011 articles) for outcomes on children >5 years of age and adults including vaccine-type nasopharyngeal carriage (VT-NP), vaccine-type invasive pneumococcal disease (VT-IPD) and syndromic pneumonia.
Results: Of 12,980 citations reviewed, we identified 21 VT-IPD, 6 VT-NP and 9 pneumonia studies. Of these 36, 21 (58%) included 3 primary doses plus PCV or pneumococcal polysaccharide vaccine (PPV23) booster schedule (3+1 or 3+PPV23), 5 (14%) 3+0, 9 (25%) 2+1 and 1 (3%) 2+0. Most (95%) were PCV7 studies. Among observational VT-IPD studies, all schedules (2+1, 3+0 and 3+1) demonstrated reductions in incidence among young adult groups. Among syndromic pneumonia observational studies (2+1, 3+0 and 3+1), only 3+1 schedules showed significant indirect impact. Of 2 VT-NP controlled trials (3+0 and 3+1) and 3 VT-NP observational studies (2+1, 3+1 and 3+PPV23), 3+1 and 3+PPV23 schedules showed significant indirect effect. The 1 study to directly compare between schedules was a VT-NP study (2+0 vs. 2+1), which found no indirect effect on older siblings and parents of vaccinated children with either schedule.
Conclusions: Indirect benefit of a 3+1 infant PCV dosing schedule has been demonstrated for VT-IPD, VT-NP and syndromic pneumonia; 2+1 and 3+0 schedules have demonstrated indirect effect only for VT-IPD. The choice of optimal infant PCV schedule is limited by data paucity on indirect effects, especially a lack of head-to-head studies and studies of PCV10 and PCV13.
Conflict of interest statement
Support for this project was provided by Program for Appropriate Technology in Health (PATH) through funding from the Global Alliance for Vaccines and Immunisation (GAVI). The views expressed by the authors do not necessarily reflect the views of GAVI and/or PATH. M.D.K. has received support from Novartis for participation on a Data and Safety Monitoring Board, meeting travel reimbursement from Pfizer and grant support from Merck. D.G.’s laboratory performs contract and or collaborative research for/with Pfizer, GlaxoSmithKline, Merck, Novartis and Sanofi Pasteur. D.G. has received travel or honorarium support for participation in external expert committees for Merck, Sanofi Pasteur, Pfizer and GlaxoSmithKline. K.O.B. received grant support from Pfizer, GlaxoSmithKline and has received travel or honorarium support for participation in external expert committees for Merck, Aventis-pasteur and GlaxoSmithKline. The authors have no other funding or conflicts of interest to declare.
Figures


References
-
- Pilishvili T, Lexau C, Farley MM, et al. Active Bacterial Core Surveillance/Emerging Infections Program Network. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41. - PubMed
-
- GAVI Alliance. Geneva, Switzerland: GAVI Alliance; 2013. Countries approved for support 2012. Available at: http://www.gavialliance.org/results/countries-approved-for-support. Accessed April 15, 2013.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

