Methods for a systematic review of pneumococcal conjugate vaccine dosing schedules
- PMID: 24336060
- PMCID: PMC3940318
- DOI: 10.1097/INF.0000000000000085
Methods for a systematic review of pneumococcal conjugate vaccine dosing schedules
Abstract
Background: Streptococcus pneumoniae causes a considerable amount of morbidity and mortality in children <5. However, pneumococcal conjugate vaccines (PCVs) can prevent much of this burden. Until recently, PCVs were mostly available only in developed countries using a variety of dosing schedules. As more lower income countries make decisions to introduce PCV into their national immunization programs, an optimal schedule with which to administer PCV has become a key policy question.
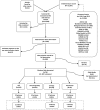
Methods: We performed a systematic review of English literature published from 1994 to 2010 on the effects of PCV dosing schedules on immunogenicity, nasopharyngeal carriage, invasive pneumococcal disease and pneumonia. Data were independently double abstracted and cleaned for analysis. Descriptive analyses were performed.
Results: We identified 12,980 citations from the literature search (12,976) and secondary means (44). Double review of titles and abstracts yielded 769 articles that underwent full data abstraction. Of these, 350 were further analyzed and are presented in separate reports in this supplement.
Conclusions: This article presents the methods utilized in our systematic review. Because of the heterogenity of the study methods of the reports identified by this review, we did not conduct formal meta-analyses. However, these methods allow us to present a full landscape of the literature on PCV dosing schedules.
Conflict of interest statement
Support for this project was provided by Program for Appropriate Technology in Health (PATH) through funding from the GAVI Alliance. The views expressed by the authors do not necessarily reflect the views of the GAVI Alliance, PATH, CDC or IVAC. M.D.K. has received support from Novartis for participation on a Data and Safety Monitoring Board, meeting travel reimbursement from Pfizer and grant support from Merck. D.G.’s laboratory performs contract and or collaborative research for/with Pfizer, GlaxoSmithKline, Merck, Novartis and Saofi Pasteur. D.G. has received travel or honorarium support for participation in external expert committees for Merck, Sanofi Pasteur, Pfizer and GlaxoSmithKline. K.O.B. had received grant support from Pfizer, GlaxoSmithKline and has received travel or honorarium support for participation in external expert committees for Merck, Aventis-pasteur and GlaxoSmithKline. The authors have no other funding or conflicts of interest to disclose.
References
-
- O’Brien KL, Wolfson LJ, Watt JP, et al. Hib and Pneumococcal Global Burden of Disease Study Team. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. - PubMed
-
- World Health Organization. Estimated Hib and pneumococcal deaths for children under 5 years of age, 2008. March 14, 2012. Available at: http://www.who.int/immunization_monitoring/burden/Pneumo_hib_estimates/e.... Accessed March 18, 2013.
-
- European Medicines Agency. Prevenar. 2011. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medici.... Accessed March 12, 2012.
-
- European Medicines Agency. Prevenar 13. 2013. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medici.... Accessed March 12, 2012.
-
- European Medicines Agency. Synflorix. 2011. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medici.... Accessed March 12, 2012.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials