Dual role of the caspase enzymes in satellite cells from aged and young subjects
- PMID: 24336075
- PMCID: PMC3877545
- DOI: 10.1038/cddis.2013.472
Dual role of the caspase enzymes in satellite cells from aged and young subjects
Abstract
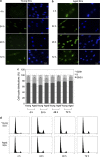
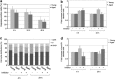
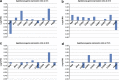
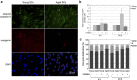
Satellite cell (SC) proliferation and differentiation have critical roles in skeletal muscle recovery after injury and adaptation in response to hypertrophic stimuli. Normal ageing hinders SC proliferation and differentiation, and is associated with increased expression of a number of pro-apoptotic factors in skeletal muscle. In light of previous studies that have demonstrated age-related altered expression of genes involved in SC antioxidant and repair activity, this investigation was aimed at evaluating the incidence of apoptotic features in human SCs. Primary cells were obtained from vastus lateralis of nine young (27.3±2.0 years old) and nine old (71.1±1.8 years old) subjects, and cultured in complete medium for analyses at 4, 24, 48, and 72 h. Apoptosis was assessed using AnnexinV/propidium iodide staining, the terminal deoxynucleotidyl transferase dUTP nick-end labelling technique, RT-PCR, DNA microarrays, flow cytometry, and immunofluorescence analysis. There was an increased rate of apoptotic cells in aged subjects at all of the experimental time points, with no direct correlation between AnnexinV-positive cells and caspase-8 activity. On the other hand, CASP2, CASP6, CASP7, and CASP9 and a number of cell death genes were upregulated in the aged SCs. Altogether, our data show age-related enhanced susceptibility of human SCs to apoptosis, which might be responsible for their reduced response to muscle damage.
Figures

Similar articles
-
Transcriptomic analysis unveils correlations between regulative apoptotic caspases and genes of cholesterol homeostasis in human brain.PLoS One. 2014 Oct 16;9(10):e110610. doi: 10.1371/journal.pone.0110610. eCollection 2014. PLoS One. 2014. PMID: 25330190 Free PMC article.
-
Aging increases the susceptibility of skeletal muscle derived satellite cells to apoptosis.Exp Gerontol. 2006 Sep;41(9):828-36. doi: 10.1016/j.exger.2006.06.053. Epub 2006 Aug 30. Exp Gerontol. 2006. PMID: 16942852
-
Twist haploinsufficiency in Saethre-Chotzen syndrome induces calvarial osteoblast apoptosis due to increased TNFalpha expression and caspase-2 activation.Hum Mol Genet. 2002 Feb 15;11(4):359-69. doi: 10.1093/hmg/11.4.359. Hum Mol Genet. 2002. PMID: 11854168
-
Apoptosis regulation by subcellular relocation of caspases.Sci Rep. 2018 Aug 15;8(1):12199. doi: 10.1038/s41598-018-30652-x. Sci Rep. 2018. PMID: 30111833 Free PMC article.
-
Danthron, an anthraquinone derivative, induces DNA damage and caspase cascades-mediated apoptosis in SNU-1 human gastric cancer cells through mitochondrial permeability transition pores and Bax-triggered pathways.Chem Res Toxicol. 2011 Jan 14;24(1):20-9. doi: 10.1021/tx100248s. Epub 2010 Dec 2. Chem Res Toxicol. 2011. PMID: 21126053
Cited by
-
The Regenerative Potential of Female Skeletal Muscle upon Hypobaric Hypoxic Exposure.Front Physiol. 2016 Jul 14;7:303. doi: 10.3389/fphys.2016.00303. eCollection 2016. Front Physiol. 2016. PMID: 27471475 Free PMC article.
-
Stem Cell Aging in Skeletal Muscle Regeneration and Disease.Int J Mol Sci. 2020 Mar 6;21(5):1830. doi: 10.3390/ijms21051830. Int J Mol Sci. 2020. PMID: 32155842 Free PMC article. Review.
-
TAK1 modulates satellite stem cell homeostasis and skeletal muscle repair.Nat Commun. 2015 Dec 9;6:10123. doi: 10.1038/ncomms10123. Nat Commun. 2015. PMID: 26648529 Free PMC article.
-
The AMPK/p27Kip1 Axis Regulates Autophagy/Apoptosis Decisions in Aged Skeletal Muscle Stem Cells.Stem Cell Reports. 2018 Aug 14;11(2):425-439. doi: 10.1016/j.stemcr.2018.06.014. Epub 2018 Jul 19. Stem Cell Reports. 2018. PMID: 30033086 Free PMC article.
-
Programmed Cell Death Genes Are Linked to Elevated Creatine Kinase Levels in Unhealthy Male Nonagenarians.Gerontology. 2016;62(5):519-29. doi: 10.1159/000443793. Epub 2016 Feb 26. Gerontology. 2016. PMID: 26913518 Free PMC article.
References
-
- Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. - PubMed
-
- Gopinath SD, Rando TA. Stem cell review series: aging of the skeletal muscle stem cell niche. Aging Cell. 2008;7:590–598. - PubMed
-
- Relaix F, Rocancourt D, Mansouri A, Buckingham MA. Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 2005;435:948–953. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

