Placenta-derived mesenchymal stem cells improve memory dysfunction in an Aβ1-42-infused mouse model of Alzheimer's disease
- PMID: 24336078
- PMCID: PMC3877561
- DOI: 10.1038/cddis.2013.490
Placenta-derived mesenchymal stem cells improve memory dysfunction in an Aβ1-42-infused mouse model of Alzheimer's disease
Abstract
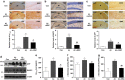
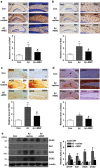
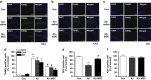
Mesenchymal stem cells (MSCs) promote functional recoveries in pathological experimental models of central nervous system (CNS) and are currently being tested in clinical trials for neurological disorders, but preventive mechanisms of placenta-derived MSCs (PD-MSCs) for Alzheimer's disease are poorly understood. Herein, we investigated the inhibitory effect of PD-MSCs on neuronal cell death and memory impairment in Aβ1-42-infused mice. After intracerebroventrical (ICV) infusion of Aβ1-42 for 14 days, the cognitive function was assessed by the Morris water maze test and passive avoidance test. Our results showed that the transplantation of PD-MSCs into Aβ1-42-infused mice significantly improved cognitive impairment, and behavioral changes attenuated the expression of APP, BACE1, and Aβ, as well as the activity of β-secretase and γ-secretase. In addition, the activation of glia cells and the expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) were inhibited by the transplantation of PD-MSCs. Furthermore, we also found that PD-MSCs downregulated the release of inflammatory cytokines as well as prevented neuronal cell death and promoted neuronal cell differentiation from neuronal progenitor cells in Aβ1-42-infused mice. These data indicate that PD-MSC mediates neuroprotection by regulating neuronal death, neurogenesis, glia cell activation in hippocampus, and altering cytokine expression, suggesting a close link between the therapeutic effects of MSCs and the damaged CNS in Alzheimer's disease.
Figures
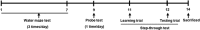


Similar articles
-
Brain-derived neurotrophic factor modified human umbilical cord mesenchymal stem cells-derived cholinergic-like neurons improve spatial learning and memory ability in Alzheimer's disease rats.Brain Res. 2019 May 1;1710:61-73. doi: 10.1016/j.brainres.2018.12.034. Epub 2018 Dec 23. Brain Res. 2019. PMID: 30586546
-
Feasibility and Efficacy of Intra-Arterial Administration of Embryonic Stem Cell Derived-Mesenchymal Stem Cells in Animal Model of Alzheimer's Disease.J Alzheimers Dis. 2020;76(4):1281-1296. doi: 10.3233/JAD-200026. J Alzheimers Dis. 2020. PMID: 32597802
-
K284-6111 prevents the amyloid beta-induced neuroinflammation and impairment of recognition memory through inhibition of NF-κB-mediated CHI3L1 expression.J Neuroinflammation. 2018 Aug 11;15(1):224. doi: 10.1186/s12974-018-1269-3. J Neuroinflammation. 2018. Retraction in: J Neuroinflammation. 2025 Feb 26;22(1):51. doi: 10.1186/s12974-025-03386-7. PMID: 30098604 Free PMC article. Retracted.
-
Prospects for the application of mesenchymal stem cells in Alzheimer's disease treatment.Life Sci. 2019 Aug 15;231:116564. doi: 10.1016/j.lfs.2019.116564. Epub 2019 Jun 13. Life Sci. 2019. PMID: 31202840 Review.
-
Effects of mesenchymal stem cells transplantation on cognitive deficits in animal models of Alzheimer's disease: A systematic review and meta-analysis.Brain Behav. 2018 Jul;8(7):e00982. doi: 10.1002/brb3.982. Epub 2018 Jun 6. Brain Behav. 2018. PMID: 29877067 Free PMC article.
Cited by
-
Stem cell therapy in Alzheimer's disease: possible benefits and limiting drawbacks.Mol Biol Rep. 2019 Feb;46(1):1425-1446. doi: 10.1007/s11033-018-4499-7. Epub 2018 Dec 18. Mol Biol Rep. 2019. PMID: 30565076 Review.
-
Stem cells technology: a powerful tool behind new brain treatments.Drug Deliv Transl Res. 2018 Oct;8(5):1564-1591. doi: 10.1007/s13346-018-0548-y. Drug Deliv Transl Res. 2018. PMID: 29916013 Review.
-
Amniotic Mesenchymal Stem Cells Decrease Aβ Deposition and Improve Memory in APP/PS1 Transgenic Mice.Neurochem Res. 2017 Aug;42(8):2191-2207. doi: 10.1007/s11064-017-2226-8. Epub 2017 Apr 10. Neurochem Res. 2017. PMID: 28397068
-
Alzheimer's Disease: Mechanism and Approach to Cell Therapy.Int J Mol Sci. 2015 Nov 4;16(11):26417-51. doi: 10.3390/ijms161125961. Int J Mol Sci. 2015. PMID: 26556341 Free PMC article. Review.
-
An update on stem cell and stem cell-derived extracellular vesicle-based therapy in the management of Alzheimer's disease.Heliyon. 2023 Jun 29;9(7):e17808. doi: 10.1016/j.heliyon.2023.e17808. eCollection 2023 Jul. Heliyon. 2023. PMID: 37449130 Free PMC article. Review.
References
-
- Momin EN, Mohyeldin A, Zaidi HA, Vela G, Quinones-Hinojosa A. Mesenchymal stem cells: new approaches for the treatment of neurological diseases. Curr Stem Cell Res Ther. 2010;5:326–344. - PubMed
-
- Peng Y, Huang S, Cheng B, Nie X, Enhe J, Feng C, et al. Mesenchymal stem cells: a revolution in therapeutic strategies of age-related diseases. Ageing Res Rev. 2013;12:103–115. - PubMed
-
- Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. - PubMed
-
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

