Lipopolysaccharide sensitized male and female juvenile brains to ionizing radiation
- PMID: 24336082
- PMCID: PMC3877553
- DOI: 10.1038/cddis.2013.482
Lipopolysaccharide sensitized male and female juvenile brains to ionizing radiation
Abstract
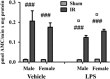
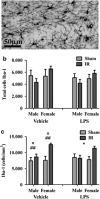
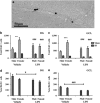
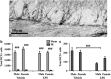
Radiotherapy is an effective tool in the treatment of pediatric malignancies but it is associated with adverse side effects, both short- and long-term. One common long-term side effect after cranial radiotherapy is cognitive impairment and this is, at least partly, thought to be caused by reduced hippocampal neurogenesis. Neuroinflammation and a perturbed microenvironment are thought to be important in the dysregulation of neurogenesis seen after irradiation (IR). We investigated the effects of a pre-existing, lipopolysaccharide (LPS)-induced systemic inflammation at the time of IR in both males and females. A single dose of 8 Gy to the brain of postnatal day 14 mice caused an upregulation of cytokines/chemokines (IL-1β, MIP-1β, IL-12, GM-CSF, MIP-1α, IL-17, CCL2 and KC) 6 h after IR, more so in females. Caspase-3 activity, reflecting apoptosis and possibly microglia activation, was elevated 6 h after IR. Females treated with LPS before IR showed a higher caspase-3 activity compared with males. During the chronic phase (3 months post IR), we found that LPS-induced inflammation at the time of IR aggravated the IR-induced injury in both male and female mice, as judged by reduced bromodeoxyuridine incorporation and neurogenesis (doublecortin-positive cells) in the hippocampus. At this late time point, the microglia density was increased by IR, more so in females, indicating long-term effects on the microenvironment. IR increased anxiety-related behavior in vehicle-, but not LPS-, treated animals. However, exploratory behavior was affected by IR in both vehicle- and LPS-treated mice. In conclusion, we found that LPS administration before IR of the young mouse brain aggravated the injury, as judged by reduced hippocampal neurogenesis. This supports the clinical practice to postpone radiotherapy if the patient shows signs of infection. Systemic inflammation is not always obvious, though, for example because of concurrent corticosteroid treatment, so careful monitoring of inflammation is warranted.
Figures


Similar articles
-
Lipopolysaccharide-induced inflammation aggravates irradiation-induced injury to the young mouse brain.Dev Neurosci. 2013;35(5):406-15. doi: 10.1159/000353820. Epub 2013 Aug 20. Dev Neurosci. 2013. PMID: 23970040
-
Different reactions to irradiation in the juvenile and adult hippocampus.Int J Radiat Biol. 2014 Sep;90(9):807-15. doi: 10.3109/09553002.2014.942015. Epub 2014 Aug 11. Int J Radiat Biol. 2014. PMID: 25004947
-
Microglia depletion and repopulation do not alter the effects of cranial irradiation on hippocampal neurogenesis.Brain Behav Immun. 2025 Jan;123:57-63. doi: 10.1016/j.bbi.2024.08.055. Epub 2024 Aug 30. Brain Behav Immun. 2025. PMID: 39218233
-
Effects of ionizing radiation on the mammalian brain.Mutat Res Rev Mutat Res. 2016 Oct-Dec;770(Pt B):219-230. doi: 10.1016/j.mrrev.2016.08.003. Epub 2016 Aug 13. Mutat Res Rev Mutat Res. 2016. PMID: 27919332 Review.
-
Brain Damage and Patterns of Neurovascular Disorder after Ionizing Irradiation. Complications in Radiotherapy and Radiation Combined Injury.Radiat Res. 2021 Jul 1;196(1):1-16. doi: 10.1667/RADE-20-00147.1. Radiat Res. 2021. PMID: 33979447 Free PMC article. Review.
Cited by
-
Research progress on mechanism and imaging of temporal lobe injury induced by radiotherapy for head and neck cancer.Eur Radiol. 2022 Jan;32(1):319-330. doi: 10.1007/s00330-021-08164-6. Epub 2021 Jul 29. Eur Radiol. 2022. PMID: 34327577 Review.
-
Radiation takes its Toll.Cancer Lett. 2015 Nov 28;368(2):238-45. doi: 10.1016/j.canlet.2015.03.031. Epub 2015 Mar 25. Cancer Lett. 2015. PMID: 25819030 Free PMC article. Review.
-
A multidisciplinary approach unravels early and persistent effects of X-ray exposure at the onset of prenatal neurogenesis.J Neurodev Disord. 2015;7(1):3. doi: 10.1186/1866-1955-7-3. Epub 2015 Jan 9. J Neurodev Disord. 2015. PMID: 26029273 Free PMC article.
-
X-ray-induced changes in the expression of inflammation-related genes in human peripheral blood.Int J Mol Sci. 2014 Oct 27;15(11):19516-34. doi: 10.3390/ijms151119516. Int J Mol Sci. 2014. PMID: 25350114 Free PMC article.
-
Cranial irradiation induces transient microglia accumulation, followed by long-lasting inflammation and loss of microglia.Oncotarget. 2016 Dec 13;7(50):82305-82323. doi: 10.18632/oncotarget.12929. Oncotarget. 2016. PMID: 27793054 Free PMC article.
References
-
- Heyman M, Gustafsson G, Kogner P.Childhood Cancer Incidence and Survival in Sweden 1984-2010 2013http://www.cceg.ki.se/documents/ChildhoodCancerIncidenceandSurvivalinSwe... .
-
- Dreifaldt AC, Carlberg M, Hardell L. Increasing incidence rates of childhood malignant diseases in Sweden during the period 1960-1998. Eur J Cancer. 2004;40:1351–1360. - PubMed
-
- Rosychuk RJ, Witol A, Wilson B, Stobart K. Central nervous system (CNS) tumor trends in children in a western Canadian province: a population-based 22-year retrospective study. J Neurol. 2012;259:1131–1136. - PubMed
-
- Lahteenmaki PM, Harila-Saari A, Pukkala EI, Kyyronen P, Salmi TT, Sankila R. Scholastic achievements of children with brain tumors at the end of comprehensive education: a nationwide, register-based study. Neurology. 2007;69:296–305. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

