Induction of necrotic cell death by oxidative stress in retinal pigment epithelial cells
- PMID: 24336085
- PMCID: PMC3877549
- DOI: 10.1038/cddis.2013.478
Induction of necrotic cell death by oxidative stress in retinal pigment epithelial cells
Abstract
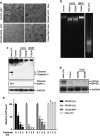
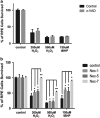
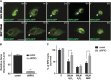
Age-related macular degeneration (AMD) is a degenerative disease of the retina and the leading cause of blindness in the elderly. Retinal pigment epithelial (RPE) cell death and the resultant photoreceptor apoptosis are characteristic of late-stage dry AMD, especially geographic atrophy (GA). Although oxidative stress and inflammation have been associated with GA, the nature and underlying mechanism for RPE cell death remains controversial, which hinders the development of targeted therapy for dry AMD. The purpose of this study is to systematically dissect the mechanism of RPE cell death induced by oxidative stress. Our results show that characteristic features of apoptosis, including DNA fragmentation, caspase 3 activation, chromatin condensation and apoptotic body formation, were not observed during RPE cell death induced by either hydrogen peroxide or tert-Butyl hydroperoxide. Instead, this kind of cell death can be prevented by RIP kinase inhibitors necrostatins but not caspase inhibitor z-VAD, suggesting necrotic feature of RPE cell death. Moreover, ATP depletion, receptor interacting protein kinase 3 (RIPK3) aggregation, nuclear and plasma membrane leakage and breakdown, which are the cardinal features of necrosis, were observed in RPE cells upon oxidative stress. Silencing of RIPK3, a key protein in necrosis, largely prevented oxidative stress-induced RPE death. The necrotic nature of RPE death is consistent with the release of nuclear protein high mobility group protein B1 into the cytoplasm and cell medium, which induces the expression of inflammatory gene TNFα in healthy RPE and THP-1 cells. Interestingly, features of pyroptosis or autophagy were not observed in oxidative stress-treated RPE cells. Our results unequivocally show that necrosis, but not apoptosis, is a major type of cell death in RPE cells in response to oxidative stress. This suggests that preventing oxidative stress-induced necrotic RPE death may be a viable approach for late-stage dry AMD.
Figures




Similar articles
-
Gossypol Acetic Acid Prevents Oxidative Stress-Induced Retinal Pigment Epithelial Necrosis by Regulating the FoxO3/Sestrin2 Pathway.Mol Cell Biol. 2015 Jun 1;35(11):1952-63. doi: 10.1128/MCB.00178-15. Epub 2015 Mar 23. Mol Cell Biol. 2015. PMID: 25802279 Free PMC article.
-
RPE necroptosis in response to oxidative stress and in AMD.Ageing Res Rev. 2015 Nov;24(Pt B):286-98. doi: 10.1016/j.arr.2015.09.002. Epub 2015 Sep 11. Ageing Res Rev. 2015. PMID: 26369358 Free PMC article. Review.
-
Oxidative stress induces ferroptotic cell death in retinal pigment epithelial cells.Exp Eye Res. 2019 Apr;181:316-324. doi: 10.1016/j.exer.2018.08.019. Epub 2018 Aug 29. Exp Eye Res. 2019. PMID: 30171859 Free PMC article.
-
Polarized human embryonic stem cell-derived retinal pigment epithelial cell monolayers have higher resistance to oxidative stress-induced cell death than nonpolarized cultures.Stem Cells Transl Med. 2015 Jan;4(1):10-20. doi: 10.5966/sctm.2014-0205. Epub 2014 Nov 19. Stem Cells Transl Med. 2015. PMID: 25411476 Free PMC article.
-
Autophagy in dry AMD: A promising therapeutic strategy for retinal pigment epithelial cell damage.Exp Eye Res. 2024 May;242:109889. doi: 10.1016/j.exer.2024.109889. Epub 2024 Apr 7. Exp Eye Res. 2024. PMID: 38593971 Review.
Cited by
-
Mechanisms of Photoreceptor Death in Retinitis Pigmentosa.Genes (Basel). 2020 Sep 24;11(10):1120. doi: 10.3390/genes11101120. Genes (Basel). 2020. PMID: 32987769 Free PMC article. Review.
-
Gossypol Acetic Acid Prevents Oxidative Stress-Induced Retinal Pigment Epithelial Necrosis by Regulating the FoxO3/Sestrin2 Pathway.Mol Cell Biol. 2015 Jun 1;35(11):1952-63. doi: 10.1128/MCB.00178-15. Epub 2015 Mar 23. Mol Cell Biol. 2015. PMID: 25802279 Free PMC article.
-
Adipose-Derived Mesenchymal Stem Cells Migrate and Rescue RPE in the Setting of Oxidative Stress.Stem Cells Int. 2018 Dec 13;2018:9682856. doi: 10.1155/2018/9682856. eCollection 2018. Stem Cells Int. 2018. PMID: 30651740 Free PMC article.
-
Comparative mechanistic study of RPE cell death induced by different oxidative stresses.Redox Biol. 2023 Sep;65:102840. doi: 10.1016/j.redox.2023.102840. Epub 2023 Aug 6. Redox Biol. 2023. PMID: 37566944 Free PMC article.
-
Quantitative estimation of intracellular oxidative stress in human tissues.Brief Bioinform. 2022 Jul 18;23(4):bbac206. doi: 10.1093/bib/bbac206. Brief Bioinform. 2022. PMID: 35653708 Free PMC article.
References
-
- Friedman DS, O'Colmain BJ, Muñoz B, Tomany SC, McCarty C, de Jong PT, et al. Prevalence of age-related macular degeneration in the United States Arch Ophthalmol 2004122p564–572. - PubMed
-
- Klein R, Klein BE, Knudtson MD, Meuer SM, Swift M, Gangnon RE. Fifteen-year cumulative incidence of age-related macular degeneration: the Beaver Dam Eye Study. Ophthalmology. 2007;114:253–262. - PubMed
-
- Age-Related Eye Disease Study 2 Research, G Lutein+zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309:2005–2015. - PubMed
-
- Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

