Assessing treatment response to interferon-β: is there a role for MRI?
- PMID: 24336144
- PMCID: PMC3902760
- DOI: 10.1212/WNL.0000000000000036
Assessing treatment response to interferon-β: is there a role for MRI?
Abstract
Objective: Interferon-β (IFN-β) has been shown to reduce relapse rates in multiple sclerosis; however, the clinical response appears to vary among individuals. Can early MRI be used to identify those patients who have a poor response to treatment?
Methods: A systematic review of studies examining differential treatment response and clinical endpoints in groups defined as responders or nonresponders to IFN-β was performed. Meta-analytic techniques were used to combine study results where appropriate.
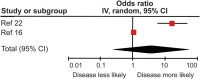
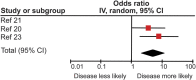
Results: Patients with MRI evidence of poor response to IFN-β treatment as defined by either ≥2 new hyperintense T2 lesions or new gadolinium-enhancing lesions had significantly increased risk of both future relapses and progression as defined by the Expanded Disability Status Scale. There appeared to be an increased risk of poor outcomes 16 years after treatment initiation in those with an initial poor response to treatment. Previous evidence has shown this not to be the case in placebo arms of clinical trials.
Conclusions: For those patients starting IFN-β, early MRI, within 6 to 24 months after starting treatment, has the potential to provide important information when counseling patients about the likelihood of future treatment failure. This can inform treatment decisions before clinical relapses or disease progression.
Figures
References
-
- PRISMS Study Group and the University of British Columbia MS/MRI Analysis Group PRISMS-4: long-term efficacy of interferon-beta-1a in relapsing MS. Neurology 2001;56:1628–1636 - PubMed
-
- The IFNB Multiple Sclerosis Study Group Interferon beta-1b is effective in relapsing-remitting multiple sclerosis: I: clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology 1993;43:655–661 - PubMed
-
- Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol 1996;39:285–294 - PubMed
-
- Kappos L, Freedman MS, Polman CH, et al. Long-term effect of early treatment with interferon beta-1b after a first clinical event suggestive of multiple sclerosis: 5-year active treatment extension of the phase 3 BENEFIT trial. Lancet Neurol 2009;8:987–997 - PubMed
-
- O'Connor P, Filippi M, Arnason B, et al. 250 microg or 500 microg interferon beta-1b versus 20 mg glatiramer acetate in relapsing-remitting multiple sclerosis: a prospective, randomised, multicentre study. Lancet Neurol 2009;8:889–897 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
