Sustained correction of motoneuron histopathology following intramuscular delivery of AAV in pompe mice
- PMID: 24336173
- PMCID: PMC3982493
- DOI: 10.1038/mt.2013.282
Sustained correction of motoneuron histopathology following intramuscular delivery of AAV in pompe mice
Abstract
Pompe disease is an autosomal recessive disorder caused by mutations in the acid-α glucosidase (GAA) gene. Lingual dysfunction is prominent but does not respond to conventional enzyme replacement therapy (ERT). Using Pompe (Gaa(-/-)) mice, we tested the hypothesis that intralingual delivery of viral vectors encoding GAA results in GAA expression and glycogen clearance in both tongue myofibers and hypoglossal (XII) motoneurons. An intralingual injection of an adeno-associated virus (AAV) vector encoding GAA (serotypes 1 or 9; 1 × 10(11) vector genomes, CMV promoter) was performed in 2-month-old Gaa(-/-) mice, and tissues were harvested 4 months later. Both serotypes robustly transduced tongue myofibers with histological confirmation of GAA expression (immunochemistry) and glycogen clearance (Period acid-Schiff stain). Both vectors also led to medullary transgene expression. GAA-positive motoneurons did not show the histopathologic features which are typical in Pompe disease and animal models. Intralingual injection with the AAV9 vector resulted in approximately threefold more GAA-positive XII motoneurons (P < 0.02 versus AAV1); the AAV9 group also gained more body weight over the course of the study (P < 0.05 versus AAV1 and sham). We conclude that intralingual injection of AAV1 or AAV9 drives persistent GAA expression in tongue myofibers and motoneurons, but AAV9 may more effectively target motoneurons.
Figures







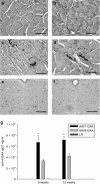
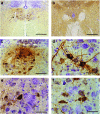
References
-
- Jones HN, Muller CW, Lin M, et al. Oropharyngeal dysphagia in infants and children with infantile Pompe disease. Dysphagia. 2010;25:277–83. - PubMed
-
- Margolis ML, Howlett P, Goldberg R, Eftychiadis A, Levine S. Obstructive sleep apnea syndrome in acid maltase deficiency. Chest. 1994;105:947–9. - PubMed
-
- Muller CW, Jones HN, OGrady G, Suarez AH, Heller JH, Kishnani PS. Language and speech function in children with infantile Pompe disease. J Ped Neurol. 2009;7:147–156.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

