Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids
- PMID: 24336213
- PMCID: PMC3961847
- DOI: 10.1038/nature12799
Mycobacteria manipulate macrophage recruitment through coordinated use of membrane lipids
Abstract
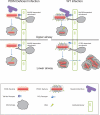
The evolutionary survival of Mycobacterium tuberculosis, the cause of human tuberculosis, depends on its ability to invade the host, replicate, and transmit infection. At its initial peripheral infection site in the distal lung airways, M. tuberculosis infects macrophages, which transport it to deeper tissues. How mycobacteria survive in these broadly microbicidal cells is an important question. Here we show in mice and zebrafish that M. tuberculosis, and its close pathogenic relative Mycobacterium marinum, preferentially recruit and infect permissive macrophages while evading microbicidal ones. This immune evasion is accomplished by using cell-surface-associated phthiocerol dimycoceroserate (PDIM) lipids to mask underlying pathogen-associated molecular patterns (PAMPs). In the absence of PDIM, these PAMPs signal a Toll-like receptor (TLR)-dependent recruitment of macrophages that produce microbicidal reactive nitrogen species. Concordantly, the related phenolic glycolipids (PGLs) promote the recruitment of permissive macrophages through a host chemokine receptor 2 (CCR2)-mediated pathway. Thus, we have identified coordinated roles for PDIM, known to be essential for mycobacterial virulence, and PGL, which (along with CCR2) is known to be associated with human tuberculosis. Our findings also suggest an explanation for the longstanding observation that M. tuberculosis initiates infection in the relatively sterile environment of the lower respiratory tract, rather than in the upper respiratory tract, where resident microflora and inhaled environmental microbes may continually recruit microbicidal macrophages through TLR-dependent signalling.
Figures
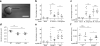


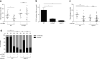










Comment in
-
Immune evasion: Mycobacteria hide from TLRs.Nat Rev Immunol. 2014 Feb;14(2):62-3. doi: 10.1038/nri3604. Epub 2013 Dec 31. Nat Rev Immunol. 2014. PMID: 24378842 No abstract available.
-
Mycobacterial lipid logic.Cell Host Microbe. 2014 Jan 15;15(1):1-2. doi: 10.1016/j.chom.2013.12.005. Cell Host Microbe. 2014. PMID: 24439891 Free PMC article.
-
Hiding behind the mycobacterial cell wall.Trends Microbiol. 2014 Mar;22(3):110-2. doi: 10.1016/j.tim.2014.01.004. Epub 2014 Jan 28. Trends Microbiol. 2014. PMID: 24485006
References
-
- Philips JA, Ernst JD. Tuberculosis Pathogenesis and Immunity. Annual Review of Pathology: Mechanisms of Disease. 2012;7:353–384. - PubMed
-
- Onwueme KC, Vos CJ, Zurita J, Ferreras JA, Quadri LEN. The dimycocerosate ester polyketide virulence factors of mycobacteria. Progress in Lipid Research. 2005;44:259–302. - PubMed
-
- Reed MB, et al. A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature. 2004;449:84–87. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

