Dynamics of yeast histone H2A and H2B phosphorylation in response to a double-strand break
- PMID: 24336221
- PMCID: PMC3889172
- DOI: 10.1038/nsmb.2737
Dynamics of yeast histone H2A and H2B phosphorylation in response to a double-strand break
Abstract
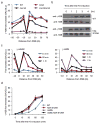
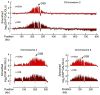
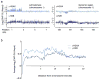
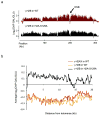
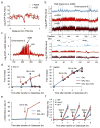
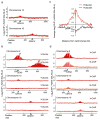
In budding yeast, a single double-strand break (DSB) triggers extensive Tel1 (ATM)- and Mec1 (ATR)-dependent phosphorylation of histone H2A around the DSB, to form γ-H2AX. We describe Mec1- and Tel1-dependent phosphorylation of histone H2B at T129. γ-H2B formation is impaired by γ-H2AX and its binding partner Rad9. High-density microarray analyses show similar γ-H2AX and γ-H2B distributions, but γ-H2B is absent near telomeres. Both γ-H2AX and γ-H2B are strongly diminished over highly transcribed regions. When transcription of GAL7, GAL10 and GAL1 genes is turned off, γ-H2AX is restored within 5 min, in a Mec1-dependent manner; after reinduction of these genes, γ-H2AX is rapidly lost. Moreover, when a DSB is induced near CEN2, γ-H2AX spreads to all other pericentromeric regions, again depending on Mec1. Our data provide new insights in the function and establishment of phosphorylation events occurring on chromatin after DSB induction.
Figures

References
-
- Harrison JC, Haber JE. Surviving the breakup: the DNA damage checkpoint. Annu Rev Genet. 2006;40:209–235. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

