Intravitreal bevacizumab (Avastin) versus triamcinolone (Volon A) for treatment of diabetic macular edema: one-year results
- PMID: 24336297
- PMCID: PMC3890767
- DOI: 10.1038/eye.2013.242
Intravitreal bevacizumab (Avastin) versus triamcinolone (Volon A) for treatment of diabetic macular edema: one-year results
Abstract
Purpose: The objective was to compare retinal morphology and function following intravitreal injections of bevacizumab (Avastin) or triamcinolone (Volon A) in patients with early diabetic macular edema (DME).
Patients and methods: The study was planned as a randomized, prospective, interventional clinical trial. A total of 30 diabetic patients with treatment-naïve, clinically significant macular edema were included in this study and randomized to two equal groups. One group initially received three injections of 2.5 mg bevacizumab in monthly intervals. The second group received a single injection of 8 mg triamcinolone, followed by two sham interventions. Functional and anatomic results were evaluated monthly using ETDRS vision charts and spectral-domain optical coherence tomography. According to the study protocol, retreatment after 3 months was dependent on functional and anatomic outcome in a PRN regimen.
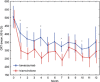
Results: Baseline best corrected visual acuity (BCVA) was 0.30 logMAR and central retinal subfield thickness (CSRT) was 505 μm in the bevacizumab group and 0.32 logMAR and 490 μm CSRT in the triamcinolone group. After 3 months, BCVA improved to 0.23 logMAR (bevacizumab) and 358 μm CRST and 0.26 logMAR (triamcinolone) and 308 μm CSRT. After 12 months, BCVA further recovered in the bevacizumab group (0.18 logMAR) but slightly decreased in the triamcinolone group (0.36 logMAR).
Conclusion: Intravitreal bevacizumab and triamcinolone are both equally effective in reducing CSRT in early DME. After 6 months, rehabilitation of vision was comparable in both treatment arms, whereas at the final follow-up at month 12, BCVA was superior in the bevacizumab than in the triamcinolone sample. This may be related to cataract development following steroid treatment, as well as to substance-specific mechanisms within the angiogenic versus the inflammatory cascade.
Figures



References
-
- Rogers SL, Tikellis G, Cheung N, Tapp R, Shaw J, Zimmet PZ, et al. Retinal arteriolar caliber predicts incident retinopathy: the Australian Diabetes, Obesity and Lifestyle (AusDiab) study. Diabetes Care. 2008;31 (4:761–763. - PubMed
-
- Focal photocoagulation treatment of diabetic macular edema. Relationship of treatment effect to fluorescein angiographic and other retinal characteristics at baseline: ETDRS report no. 19. Early Treatment Diabetic Retinopathy Study Research Group. Arch Ophthalmol. 1995;113 (9:1144–1155. - PubMed
-
- Noma H, Funatsu H, Yamasaki M, Tsukamoto H, Mimura T, Sone T, et al. Pathogenesis of macular edema with branch retinal vein occlusion and intraocular levels of vascular endothelial growth factor and interleukin-6. Am J Ophthalmol. 2005;140 (2:256–261. - PubMed
-
- Jaissle GB, Szurman P, Bartz-Schmidt KU. [Recommendation for the implementation of intravitreal injections—statement of the German Retina Society, the German Society of Ophthalmology (DOG) and the German Professional Association of Ophthalmologists (BVA)] Klin Monbl Augenheilkd. 2005;222 (5:390–395. - PubMed
-
- Klein R, Moss SE, Klein BE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. XI. The incidence of macular edema. Ophthalmology. 1989;96 (10:1501–1510. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

