Unbiased estimation of chloroplast number in mesophyll cells: advantage of a genuine three-dimensional approach
- PMID: 24336344
- PMCID: PMC3904715
- DOI: 10.1093/jxb/ert407
Unbiased estimation of chloroplast number in mesophyll cells: advantage of a genuine three-dimensional approach
Abstract
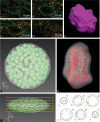
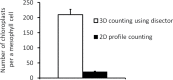
Chloroplast number per cell is a frequently examined quantitative anatomical parameter, often estimated by counting chloroplast profiles in two-dimensional (2D) sections of mesophyll cells. However, a mesophyll cell is a three-dimensional (3D) structure and this has to be taken into account when quantifying its internal structure. We compared 2D and 3D approaches to chloroplast counting from different points of view: (i) in practical measurements of mesophyll cells of Norway spruce needles, (ii) in a 3D model of a mesophyll cell with chloroplasts, and (iii) using a theoretical analysis. We applied, for the first time, the stereological method of an optical disector based on counting chloroplasts in stacks of spruce needle optical cross-sections acquired by confocal laser-scanning microscopy. This estimate was compared with counting chloroplast profiles in 2D sections from the same stacks of sections. Comparing practical measurements of mesophyll cells, calculations performed in a 3D model of a cell with chloroplasts as well as a theoretical analysis showed that the 2D approach yielded biased results, while the underestimation could be up to 10-fold. We proved that the frequently used method for counting chloroplasts in a mesophyll cell by counting their profiles in 2D sections did not give correct results. We concluded that the present disector method can be efficiently used for unbiased estimation of chloroplast number per mesophyll cell. This should be the method of choice, especially in coniferous needles and leaves with mesophyll cells with lignified cell walls where maceration methods are difficult or impossible to use.
Keywords: Chloroplast counting; Norway spruce (Picea abies L. Karst.); confocal microscopy; coniferous needle structure; disector method; mesophyll; profile counting; stereology..
Figures




References
-
- Adachi S, Nakae T, Uchida M, et al. 2013. The mesophyll anatomy enhancing CO2 diffusion is a key trait for improving rice photosynthesis. Journal of Experimental Botany 64, 1061–1072 - PubMed
-
- Albrechtová J, Kubínová L. 1991. Quantitative analysis of the structure of etiolated barley leaf using stereological methods. Journal of Experimental Botany 42, 1311–1314
-
- Albrechtová J, Janáček J, Lhotáková Z, Radochová B, Kubínová L. 2007. Novel efficient methods for measuring mesophyll anatomical characteristics from fresh thick sections using stereology and confocal microscopy: application on acid rain-treated Norway spruce needles. Journal of Experimental Botany 58, 1451–1461 - PubMed
-
- Bockers M, Čapková V, Tichá I, Schafer C. 1997. Growth at high CO2 affects the chloroplast number but not the photosynthetic efficiency of photoautotrophic Marchantia polymorpha culture cells. Plant Cell Tissue and Organ Culture 48, 103–110
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

