Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma
- PMID: 24336570
- PMCID: PMC3998672
- DOI: 10.1126/science.1239947
Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma
Abstract
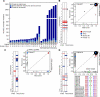
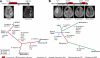
Tumor recurrence is a leading cause of cancer mortality. Therapies for recurrent disease may fail, at least in part, because the genomic alterations driving the growth of recurrences are distinct from those in the initial tumor. To explore this hypothesis, we sequenced the exomes of 23 initial low-grade gliomas and recurrent tumors resected from the same patients. In 43% of cases, at least half of the mutations in the initial tumor were undetected at recurrence, including driver mutations in TP53, ATRX, SMARCA4, and BRAF; this suggests that recurrent tumors are often seeded by cells derived from the initial tumor at a very early stage of their evolution. Notably, tumors from 6 of 10 patients treated with the chemotherapeutic drug temozolomide (TMZ) followed an alternative evolutionary path to high-grade glioma. At recurrence, these tumors were hypermutated and harbored driver mutations in the RB (retinoblastoma) and Akt-mTOR (mammalian target of rapamycin) pathways that bore the signature of TMZ-induced mutagenesis.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- K08 NS079485/NS/NINDS NIH HHS/United States
- P01CA81403/CA/NCI NIH HHS/United States
- P01 CA081403/CA/NCI NIH HHS/United States
- R01CA169316-01/CA/NCI NIH HHS/United States
- R01 CA169316/CA/NCI NIH HHS/United States
- K08NS079485/NS/NINDS NIH HHS/United States
- P50 CA097257/CA/NCI NIH HHS/United States
- P50CA097257/CA/NCI NIH HHS/United States
- T32 GM008568/GM/NIGMS NIH HHS/United States
- R01CA163336/CA/NCI NIH HHS/United States
- T32 CA128583/CA/NCI NIH HHS/United States
- R01 CA163336/CA/NCI NIH HHS/United States
- T32GM008568/GM/NIGMS NIH HHS/United States
- R25 NS070680/NS/NINDS NIH HHS/United States
- R25NS070680/NS/NINDS NIH HHS/United States
- P30 CA082103/CA/NCI NIH HHS/United States
- P30CA82103/CA/NCI NIH HHS/United States
- 1T32CA15102201/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

