The Coffin-Lowry syndrome-associated protein RSK2 regulates neurite outgrowth through phosphorylation of phospholipase D1 (PLD1) and synthesis of phosphatidic acid
- PMID: 24336713
- PMCID: PMC6618760
- DOI: 10.1523/JNEUROSCI.2283-13.2013
The Coffin-Lowry syndrome-associated protein RSK2 regulates neurite outgrowth through phosphorylation of phospholipase D1 (PLD1) and synthesis of phosphatidic acid
Abstract
More than 80 human X-linked genes have been associated with mental retardation and deficits in learning and memory. However, most of the identified mutations induce limited morphological alterations in brain organization and the molecular bases underlying neuronal clinical features remain elusive. We show here that neurons cultured from mice lacking ribosomal S6 kinase 2 (Rsk2), a model for the Coffin-Lowry syndrome (CLS), exhibit a significant delay in growth in a similar way to that shown by neurons cultured from phospholipase D1 (Pld1) knock-out mice. We found that gene silencing of Pld1 or Rsk2 as well as acute pharmacological inhibition of PLD1 or RSK2 in PC12 cells strongly impaired neuronal growth factor (NGF)-induced neurite outgrowth. Expression of a phosphomimetic PLD1 mutant rescued the inhibition of neurite outgrowth in PC12 cells silenced for RSK2, revealing that PLD1 is a major target for RSK2 in neurite formation. NGF-triggered RSK2-dependent phosphorylation of PLD1 led to its activation and the synthesis of phosphatidic acid at sites of neurite growth. Additionally, total internal reflection fluorescence microscopy experiments revealed that RSK2 and PLD1 positively control fusion of tetanus neurotoxin insensitive vesicle-associated membrane protein (TiVAMP)/VAMP-7 vesicles at sites of neurite outgrowth. We propose that the loss of function mutations in RSK2 that leads to CLS and neuronal deficits are related to defects in neuronal growth due to impaired RSK2-dependent PLD1 activity resulting in a reduced vesicle fusion rate and membrane supply.
Figures

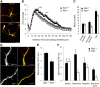

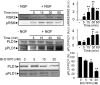
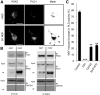
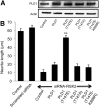


Similar articles
-
The Coffin-Lowry syndrome-associated protein RSK2 is implicated in calcium-regulated exocytosis through the regulation of PLD1.Proc Natl Acad Sci U S A. 2008 Jun 17;105(24):8434-9. doi: 10.1073/pnas.0710676105. Epub 2008 Jun 11. Proc Natl Acad Sci U S A. 2008. PMID: 18550821 Free PMC article.
-
The Coffin-Lowry syndrome-associated protein RSK2 controls neuroendocrine secretion through the regulation of phospholipase D1 at the exocytotic sites.Ann N Y Acad Sci. 2009 Jan;1152:201-8. doi: 10.1111/j.1749-6632.2008.04001.x. Ann N Y Acad Sci. 2009. PMID: 19161391
-
Defective synaptic transmission and structure in the dentate gyrus and selective fear memory impairment in the Rsk2 mutant mouse model of Coffin-Lowry syndrome.Neurobiol Dis. 2013 Oct;58:156-68. doi: 10.1016/j.nbd.2013.05.016. Epub 2013 Jun 3. Neurobiol Dis. 2013. PMID: 23742761
-
The Coffin-Lowry syndrome-associated protein RSK2 and neurosecretion.Cell Mol Neurobiol. 2010 Nov;30(8):1401-6. doi: 10.1007/s10571-010-9578-9. Cell Mol Neurobiol. 2010. PMID: 21061166 Free PMC article. Review.
-
Coffin-Lowry syndrome.Eur J Hum Genet. 2010 Jun;18(6):627-33. doi: 10.1038/ejhg.2009.189. Epub 2009 Nov 4. Eur J Hum Genet. 2010. PMID: 19888300 Free PMC article. Review.
Cited by
-
Role of tetanus neurotoxin insensitive vesicle-associated membrane protein in membrane domains transport and homeostasis.Cell Logist. 2015 Apr 29;5(1):e1025182. doi: 10.1080/21592799.2015.1025182. eCollection 2015 Jan-Mar. Cell Logist. 2015. PMID: 26196023 Free PMC article.
-
Animal Models for Coffin-Lowry Syndrome: RSK2 and Nervous System Dysfunction.Front Behav Neurosci. 2018 May 23;12:106. doi: 10.3389/fnbeh.2018.00106. eCollection 2018. Front Behav Neurosci. 2018. PMID: 29875643 Free PMC article. Review.
-
Phosphatidic Acid: From Pleiotropic Functions to Neuronal Pathology.Front Cell Neurosci. 2019 Jan 23;13:2. doi: 10.3389/fncel.2019.00002. eCollection 2019. Front Cell Neurosci. 2019. PMID: 30728767 Free PMC article. Review.
-
Cellular and physiological roles for phospholipase D1 in cancer.J Biol Chem. 2014 Aug 15;289(33):22567-22574. doi: 10.1074/jbc.R114.576876. Epub 2014 Jul 2. J Biol Chem. 2014. PMID: 24990946 Free PMC article. Review.
-
Astrocyte-derived phosphatidic acid promotes dendritic branching.Sci Rep. 2016 Feb 17;6:21096. doi: 10.1038/srep21096. Sci Rep. 2016. PMID: 26883475 Free PMC article.
References
-
- Cai D, Zhong M, Wang R, Netzer WJ, Shields D, Zheng H, Sisodia SS, Foster DA, Gorelick FS, Xu H, Greengard P. Phospholipase D1 corrects impaired betaAPP trafficking and neurite outgrowth in familial Alzheimer's disease-linked presenilin-1 mutant neurons. Proc Natl Acad Sci U S A. 2006;103:1936–1940. doi: 10.1073/pnas.0510710103. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
