Motor axon regeneration and muscle reinnervation in young adult and aged animals
- PMID: 24336714
- PMCID: PMC6618761
- DOI: 10.1523/JNEUROSCI.4067-13.2013
Motor axon regeneration and muscle reinnervation in young adult and aged animals
Abstract
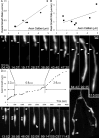
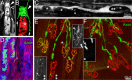
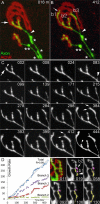
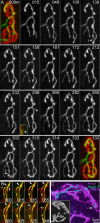
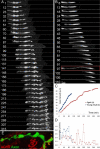
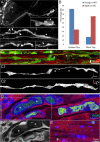
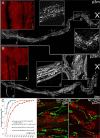
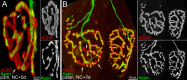
Injuries to peripheral nerves can cause paralysis and sensory disturbances, but such functional impairments are often short lived because of efficient regeneration of damaged axons. The time required for functional recovery, however, increases with advancing age (Verdú et al., 2000; Kawabuchi et al., 2011). Incomplete or delayed recovery after peripheral nerve damage is a major health concern in the aging population because it can severely restrict a person's mobility and independence. A variety of possible causes have been suggested to explain why nervous systems in aged individuals recover more slowly from nerve damage. Potential causes include age-related declines in the regenerative potential of peripheral axons and decreases in the supply or responsivity to trophic and/or tropic factors. However, there have been few direct analyses of age-related axon regeneration. Our aim here was to observe axons directly in young and old mice as they regenerate and ultimately reoccupy denervated neuromuscular synaptic sites to learn what changes in this process are age related. We find that damaged nerves in aged animals clear debris more slowly than nerves in young animals and that the greater number of obstructions regenerating axons encounter in the endoneurial tubes of old animals give rise to slower regeneration. Surprisingly, however, axons from aged animals regenerate quickly when not confronted by debris and reoccupy neuromuscular junction sites efficiently. These results imply that facilitating clearance of axon debris might be a good target for the treatment of nerve injury in the aged.
Keywords: aging; axon regeneration; in vivo imaging; neuromuscular system; self-avoidance; synapse reinnervation.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
