Multimodal integration of self-motion cues in the vestibular system: active versus passive translations
- PMID: 24336720
- PMCID: PMC3858625
- DOI: 10.1523/JNEUROSCI.3051-13.2013
Multimodal integration of self-motion cues in the vestibular system: active versus passive translations
Abstract
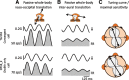
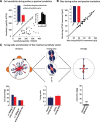
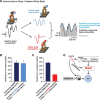
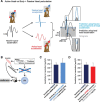
The ability to keep track of where we are going as we navigate through our environment requires knowledge of our ongoing location and orientation. In response to passively applied motion, the otolith organs of the vestibular system encode changes in the velocity and direction of linear self-motion (i.e., heading). When self-motion is voluntarily generated, proprioceptive and motor efference copy information is also available to contribute to the brain's internal representation of current heading direction and speed. However to date, how the brain integrates these extra-vestibular cues with otolith signals during active linear self-motion remains unknown. Here, to address this question, we compared the responses of macaque vestibular neurons during active and passive translations. Single-unit recordings were made from a subgroup of neurons at the first central stage of sensory processing in the vestibular pathways involved in postural control and the computation of self-motion perception. Neurons responded far less robustly to otolith stimulation during self-generated than passive head translations. Yet, the mechanism underlying the marked cancellation of otolith signals did not affect other characteristics of neuronal responses (i.e., baseline firing rate, tuning ratio, orientation of maximal sensitivity vector). Transiently applied perturbations during active motion further established that an otolith cancellation signal was only gated in conditions where proprioceptive sensory feedback matched the motor-based expectation. Together our results have important implications for understanding the brain's ability to ensure accurate postural and motor control, as well as perceptual stability, during active self-motion.
Keywords: corollary discharge; efference copy; head motion; neuron; otolith; proprioception.
Figures


References
-
- Angelaki DE, Dickman JD. Spatiotemporal processing of linear acceleration: primary afferent and central vestibular neuron responses. J Neurophysiol. 2000;84:2113–2132. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
