Progranulin deficiency promotes post-ischemic blood-brain barrier disruption
- PMID: 24336722
- PMCID: PMC3858627
- DOI: 10.1523/JNEUROSCI.4318-13.2013
Progranulin deficiency promotes post-ischemic blood-brain barrier disruption
Abstract
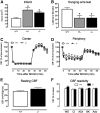
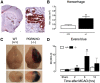
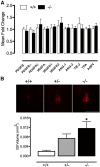
Loss-of-function mutations of progranulin (PGRN) have been linked to frontotemporal dementia, but little is known about the effects of PGRN deficiency on the brain in health and disease. PGRN has been implicated in neurovascular development, inflammation, and Wnt signaling, a pathway involved in the formation of the blood-brain barrier (BBB). Because BBB alterations and inflammation contribute to ischemic brain injury, we examined the role of PGRN in the brain damage produced by ischemia-reperfusion. PGRN(+/-) and PGRN(-/-) mice underwent middle cerebral artery occlusion (MCAO) with monitoring of cerebral blood flow. Infarct volume and motor deficits were assessed 72 h later. Post-ischemic inflammation was examined by expression of inflammatory genes and flow cytometry. BBB structure and permeability were examined by electron microscopy (EM) and Evans blue (EB) extravasation, respectively. MCAO resulted in ~60% larger infarcts in PGRN(+/-) and PGRN(-/-) mice, an effect independent of hemodynamic factors or post-ischemic inflammation. Rather, massive hemorrhages and post-ischemic BBB disruption were observed, unrelated to degradation of tight junction (TJ) proteins or matrix metalloproteinases (MMPs). By EM, TJ were 30-52% shorter, fewer, and less interlocking, suggesting a weaker seal between endothelial cells. Intracerebral injection of platelet-derived growth factor-CC (PDGF-CC), which increases BBB permeability, resulted in a more severe BBB breakdown in PGRN(+/-) and PGRN(-/-) than wild-type mice. We describe a previously unrecognized involvement of PGRN in the expression of key ultrastructural features of the BBB. Such a novel vasoprotective role of PGRN may contribute to brain dysfunction and damage in conditions associated with reduced PGRN function.
Keywords: blood–brain barrier; frontotemporal dementia; neurovascular unit; progranulin; stroke.
Figures






References
-
- Almeida S, Zhang Z, Coppola G, Mao W, Futai K, Karydas A, Geschwind MD, Tartaglia MC, Gao F, Gianni D, Sena-Esteves M, Geschwind DH, Miller BL, Farese RV, Jr, Gao F-B. Induced pluripotent stem cell models of progranulin-deficient frontotemporal dementia uncover specific reversible neuronal defects. Cell Rep. 2012;2:789–798. doi: 10.1016/j.celrep.2012.09.007. - DOI - PMC - PubMed
-
- Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Berger Z, Eriksen J, Robinson T, Zehr C, Dickey CA, Crook R, McGowan E, Mann D, Boeve B, Feldman H, Hutton M. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. doi: 10.1038/nature05016. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
