Altered structural and functional synaptic plasticity with motor skill learning in a mouse model of fragile X syndrome
- PMID: 24336735
- PMCID: PMC3858638
- DOI: 10.1523/JNEUROSCI.2514-13.2013
Altered structural and functional synaptic plasticity with motor skill learning in a mouse model of fragile X syndrome
Abstract
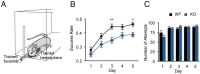
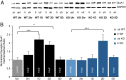
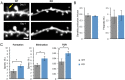
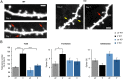
Fragile X syndrome (FXS) is the most common inherited intellectual disability. FXS results from a mutation that causes silencing of the FMR1 gene, which encodes the fragile X mental retardation protein. Patients with FXS exhibit a range of neurological deficits, including motor skill deficits. Here, we have investigated motor skill learning and its synaptic correlates in the fmr1 knock-out (KO) mouse. We find that fmr1 KO mice have impaired motor skill learning of a forelimb-reaching task, compared with their wild-type (WT) littermate controls. Electrophysiological recordings from the forelimb region of the primary motor cortex demonstrated reduced, training-induced synaptic strengthening in the trained hemisphere. Moreover, long-term potentiation (LTP) is impaired in the fmr1 KO mouse, and motor skill training does not occlude LTP as it does in the WT mice. Whereas motor skill training induces an increase of synaptic AMPA-type glutamate receptor subunit 1 (GluA1), there is a delay in GluA1 increase in the trained hemisphere of the fmr1 KO mice. Using transcranial in vivo multiphoton microscopy, we find that fmr1 KO mice have similar spine density but increased dendritic spine turnover compared with WT mice. Finally, we report that motor skill training-induced formation of dendritic spines is impaired in fmr1 KO mice. We conclude that FMRP plays a role in motor skill learning and that reduced functional and structural synaptic plasticity might underlie the behavioral deficit in the fmr1 KO mouse.
Figures


Similar articles
-
Impaired AMPARs Translocation into Dendritic Spines with Motor Skill Learning in the Fragile X Mouse Model.eNeuro. 2023 Mar 27;10(3):ENEURO.0364-22.2023. doi: 10.1523/ENEURO.0364-22.2023. Print 2023 Mar. eNeuro. 2023. PMID: 36898833 Free PMC article.
-
Astrocytic Contributions to Synaptic and Learning Abnormalities in a Mouse Model of Fragile X Syndrome.Biol Psychiatry. 2017 Jul 15;82(2):139-149. doi: 10.1016/j.biopsych.2016.08.036. Epub 2016 Sep 13. Biol Psychiatry. 2017. PMID: 27865451 Free PMC article.
-
Elevated progranulin contributes to synaptic and learning deficit due to loss of fragile X mental retardation protein.Brain. 2017 Dec 1;140(12):3215-3232. doi: 10.1093/brain/awx265. Brain. 2017. PMID: 29096020
-
BDNF in fragile X syndrome.Neuropharmacology. 2014 Jan;76 Pt C:729-36. doi: 10.1016/j.neuropharm.2013.05.018. Epub 2013 May 29. Neuropharmacology. 2014. PMID: 23727436 Review.
-
The trouble with spines in fragile X syndrome: density, maturity and plasticity.Neuroscience. 2013 Oct 22;251:120-8. doi: 10.1016/j.neuroscience.2012.03.049. Epub 2012 Apr 20. Neuroscience. 2013. PMID: 22522472 Free PMC article. Review.
Cited by
-
Dendritic Spine Plasticity: Function and Mechanisms.Front Synaptic Neurosci. 2020 Aug 28;12:36. doi: 10.3389/fnsyn.2020.00036. eCollection 2020. Front Synaptic Neurosci. 2020. PMID: 32982715 Free PMC article.
-
Reward-Related Expectations Trigger Dendritic Spine Plasticity in the Mouse Ventrolateral Orbitofrontal Cortex.J Neurosci. 2019 Jun 5;39(23):4595-4605. doi: 10.1523/JNEUROSCI.2031-18.2019. Epub 2019 Apr 2. J Neurosci. 2019. PMID: 30940719 Free PMC article.
-
Intrinsic Spine Dynamics Are Critical for Recurrent Network Learning in Models With and Without Autism Spectrum Disorder.Front Comput Neurosci. 2019 Jun 13;13:38. doi: 10.3389/fncom.2019.00038. eCollection 2019. Front Comput Neurosci. 2019. PMID: 31263407 Free PMC article.
-
Implantation of a Cranial Window for Repeated In Vivo Imaging in Awake Mice.J Vis Exp. 2021 Jun 22;(172):10.3791/62633. doi: 10.3791/62633. J Vis Exp. 2021. PMID: 34251372 Free PMC article.
-
Glutamatergic Dysfunction and Synaptic Ultrastructural Alterations in Schizophrenia and Autism Spectrum Disorder: Evidence from Human and Rodent Studies.Int J Mol Sci. 2020 Dec 23;22(1):59. doi: 10.3390/ijms22010059. Int J Mol Sci. 2020. PMID: 33374598 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
