Telaprevir in the treatment of acute hepatitis C virus infection in HIV-infected men
- PMID: 24336914
- PMCID: PMC3935497
- DOI: 10.1093/cid/cit799
Telaprevir in the treatment of acute hepatitis C virus infection in HIV-infected men
Abstract
Background: There is an international epidemic of hepatitis C virus (HCV) infection among human immunodeficiency virus (HIV)-infected men who have sex with men. Sustained virologic response (SVR) rates with pegylated interferon and ribavirin treatment are higher in these men during acute HCV than during chronic HCV, but treatment is still lengthy and SVR rates are suboptimal.
Methods: We performed a pilot study of combination therapy with telaprevir, pegylated interferon, and ribavirin in acute genotype 1 HCV infection in HIV-infected men. Men who were treated prior to the availability of, or ineligible for, telaprevir were the comparator group. The primary endpoint was SVR12, defined as an HCV viral load <5 IU/mL at least 12 weeks after completing treatment.
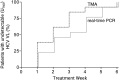
Results: In the telaprevir group, 84% (16/19) of men achieved SVR12 vs 63% (30/48) in the comparator group. Among men with SVR, median time to undetectable viral load was week 2 in the telaprevir group vs week 4 in the comparator group, and 94% vs 53% had undetectable viral loads at week 4. Most patients (81%) who achieved SVR in the telaprevir group received ≤12 weeks of treatment and there were no relapses after treatment. The overall safety profile was similar to that known for telaprevir-based regimens.
Conclusions: Incorporating telaprevir into treatment of acute genotype 1 HCV in HIV-infected men halved the treatment duration and increased the SVR rate. Larger studies should be done to confirm these findings. Clinicians should be alert to detect acute HCV infection of HIV-infected men to take advantage of this effective therapy and decrease further transmission in this epidemic.
Keywords: HIV infection; acute HCV; men who have sex with men; telaprevir; treatment.
Figures



References
-
- Chak E, Talal AH, Sherman KE, Schiff ER, Saab S. Hepatitis C virus infection in USA: an estimate of true prevalence. Liver Int. 2011;31:1090–101. - PubMed
-
- Jaeckel E, Cornberg M, Wedemeyer H, et al. Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med. 2001;345:1452–7. - PubMed
-
- Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–82. - PubMed
-
- Gilleece YC, Browne RE, Asboe D, et al. Transmission of hepatitis C virus among HIV-positive homosexual men and response to a 24-week course of pegylated interferon and ribavirin. J Acquir Immune Defic Syndr. 2005;40:41–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

