Role of the Apt1 protein in polysaccharide secretion by Cryptococcus neoformans
- PMID: 24337112
- PMCID: PMC4054279
- DOI: 10.1128/EC.00273-13
Role of the Apt1 protein in polysaccharide secretion by Cryptococcus neoformans
Abstract
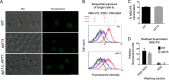
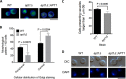
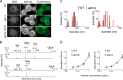
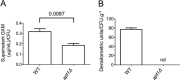
Flippases are key regulators of membrane asymmetry and secretory mechanisms. Vesicular polysaccharide secretion is essential for the pathogenic mechanisms of Cryptococcus neoformans. On the basis of the observations that flippases are required for polysaccharide secretion in plants and the putative Apt1 flippase is required for cryptococcal virulence, we analyzed the role of this enzyme in polysaccharide release by C. neoformans, using a previously characterized apt1Δ mutant. Mutant and wild-type (WT) cells shared important phenotypic characteristics, including capsule morphology and dimensions, glucuronoxylomannan (GXM) composition, molecular size, and serological properties. The apt1Δ mutant, however, produced extracellular vesicles (EVs) with a lower GXM content and different size distribution in comparison with those of WT cells. Our data also suggested a defective intracellular GXM synthesis in mutant cells, in addition to changes in the architecture of the Golgi apparatus. These findings were correlated with diminished GXM production during in vitro growth, macrophage infection, and lung colonization. This phenotype was associated with decreased survival of the mutant in the lungs of infected mice, reduced induction of interleukin-6 (IL-6) cytokine levels, and inefficacy in colonization of the brain. Taken together, our results indicate that the lack of APT1 caused defects in both GXM synthesis and vesicular export to the extracellular milieu by C. neoformans via processes that are apparently related to the pathogenic mechanisms used by this fungus during animal infection.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures




References
-
- Schekman RW. 1994. Regulation of membrane traffic in the secretory pathway. Harvey Lect. 90:41–57 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

