IL-32 promotes angiogenesis
- PMID: 24337385
- PMCID: PMC4007307
- DOI: 10.4049/jimmunol.1202802
IL-32 promotes angiogenesis
Abstract
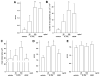
IL-32 is a multifaceted cytokine with a role in infections, autoimmune diseases, and cancer, and it exerts diverse functions, including aggravation of inflammation and inhibition of virus propagation. We previously identified IL-32 as a critical regulator of endothelial cell (EC) functions, and we now reveal that IL-32 also possesses angiogenic properties. The hyperproliferative ECs of human pulmonary arterial hypertension and glioblastoma multiforme exhibited a markedly increased abundance of IL-32, and, significantly, the cytokine colocalized with integrin αVβ3. Vascular endothelial growth factor (VEGF) receptor blockade, which resulted in EC hyperproliferation, increased IL-32 three-fold. Small interfering RNA-mediated silencing of IL-32 negated the 58% proliferation of ECs that occurred within 24 h in scrambled-transfected controls. Reduction of IL-32 neither affected apoptosis (insignificant changes in Bak-1, Bcl-2, Bcl-xL, lactate dehydrogenase, annexin V, and propidium iodide) nor VEGF or TGF-β levels, but siIL-32-transfected adult and neonatal ECs produced up to 61% less NO, IL-8, and matrix metalloproteinase-9, and up to 3-fold more activin A and endostatin. In coculture-based angiogenesis assays, IL-32γ dose-dependently increased tube formation up to 3-fold; an αVβ3 inhibitor prevented this activity and reduced IL-32γ-induced IL-8 by 85%. In matrigel plugs loaded with IL-32γ, VEGF, or vehicle and injected into live mice, we observed the anticipated VEGF-induced increase in neocapillarization (8-fold versus vehicle), but unexpectedly, IL-32γ was equally angiogenic. A second signal such as IFN-γ was required to render cells responsive to exogenous IL-32γ; importantly, this was confirmed using a completely synthetic preparation of IL-32γ. In summary, we add angiogenic properties that are mediated by integrin αVβ3 but VEGF-independent to the portfolio of IL-32, implicating a role for this versatile cytokine in pulmonary arterial hypertension and neoplastic diseases.
Figures







References
-
- Kim SH, Han SY, Azam T, Yoon DY, Dinarello CA. Interleukin-32: a cytokine and inducer of TNFalpha. Immunity. 2005;22:131–142. - PubMed
-
- Goda C, Kanaji T, Kanaji S, Tanaka G, Arima K, Ohno S, Izuhara K. Involvement of IL-32 in activation-induced cell death in T cells. Int Immunol. 2006;18:233–240. - PubMed
-
- Imaeda H, Andoh A, Aomatsu T, Osaki R, Bamba S, Inatomi O, Shimizu T, Fujiyama Y. A new isoform of interleukin-32 suppresses IL-8 mrna expression in the intestinal epithelial cell line ht-29. Mol Med Report. 2011;4:483–487. - PubMed
-
- Heinhuis B, Netea MG, van den Berg WB, Dinarello CA, Joosten LA. Interleukin-32: A predominantly intracellular proinflammatory mediator that controls cell activation and cell death. Cytokine 2012 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

