How to determine bortezomib-based regimen for elderly patients with multiple myeloma: PAD versus CBd, an observational study
- PMID: 24337419
- PMCID: PMC11823976
- DOI: 10.1007/s00432-013-1570-6
How to determine bortezomib-based regimen for elderly patients with multiple myeloma: PAD versus CBd, an observational study
Abstract
Background: This was an open-label, observational, prospective assessment. We conducted an analysis of the impact of bortezomib-based therapy (PAD: bortezomib, doxorubicin, high-dose dexamethasone vs. CBd: cyclophosphamide bortezomib, low-dose dexamethasone) on the survival rates and adverse events in elderly patients with newly diagnosed multiple myeloma (MM).
Methods: Out of 303 patients, 128 received the PAD regimen and the other 175 patients received the CBd induction therapy (age 65-89 years). Baseline patient characteristics between the two cohorts were balanced in age (P = 0.69), international staging system (ISS) prognostic stages (P = 0.90), serum calcium (P = 0.70), and serum creatinine (P = 0.52).
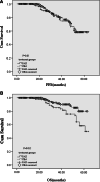
Results: Overall response (OS) after the induction chemotherapy was achieved in 214 of 303 patients (70.6 %), with no significant differences observed between the two treatment groups (71.9 vs. 69.7 %, P = 0.68). Patients with ISS stage 2 reached the same 5-year OS advantages compared to patients with ISS stage 1, because they received bortezomib-based PAD or CBd treatments. Patients receiving CBd protocol gained similar satisfactory progression-free survival (PFS) results when compared to the PAD regimen group: PFS at 5 years reached 58.2 versus 58.9 % (P = 0.85). Five-year OS in the CBd arm had significant advantages compared to the PAD group, 79.9 versus 49.9 % (P < 0.05). The overall safety profiles showed that 26 of 128 (20.3 %) patients died in the PAD arm, while 13 of 175 patients died (7.4 %) in the CBd group (P < 0.01). Similarly, the PAD arm had a higher serious infection rate than that of the CBd arm (39.2 vs. 13.1 %, P < 0.01).
Conclusions: Bortezomib benefits elderly patients with newly diagnosed MM; they achieve satisfactory treatment responses and survival advantages. Further, patients treated with CBd have superior treatment advantages, with a predictable safety profile, when compared to the PAD regimen.
Conflict of interest statement
I would like to declare on behalf of my co-authors that this work is original research that has not been published previously and is not under consideration for publication elsewhere either in part of in whole. All the authors listed have approved the manuscript that is enclosed.
Figures
Similar articles
-
The clinical effectiveness and cost-effectiveness of bortezomib and thalidomide in combination regimens with an alkylating agent and a corticosteroid for the first-line treatment of multiple myeloma: a systematic review and economic evaluation.Health Technol Assess. 2011 Dec;15(41):1-204. doi: 10.3310/hta15410. Health Technol Assess. 2011. PMID: 22146234 Free PMC article.
-
A randomized phase II, open-label and multicenter study of combination regimens of bortezomib at two doses by subcutaneous injection for newly diagnosed multiple myeloma patients.J Cancer Res Clin Oncol. 2019 Sep;145(9):2343-2355. doi: 10.1007/s00432-019-02967-3. Epub 2019 Jul 6. J Cancer Res Clin Oncol. 2019. PMID: 31280348 Free PMC article. Clinical Trial.
-
Optimisation of chemotherapy and radiotherapy for untreated Hodgkin lymphoma patients with respect to second malignant neoplasms, overall and progression-free survival: individual participant data analysis.Cochrane Database Syst Rev. 2017 Sep 13;9(9):CD008814. doi: 10.1002/14651858.CD008814.pub2. Cochrane Database Syst Rev. 2017. PMID: 28901021 Free PMC article.
-
Bortezomib for the treatment of multiple myeloma.Cochrane Database Syst Rev. 2016 Apr 20;4(4):CD010816. doi: 10.1002/14651858.CD010816.pub2. Cochrane Database Syst Rev. 2016. PMID: 27096326 Free PMC article.
-
Bendamustine and prednisone in combination with bortezomib (BPV) in the treatment of patients with newly diagnosed/untreated multiple myeloma.J Cancer Res Clin Oncol. 2014 Nov;140(11):1947-56. doi: 10.1007/s00432-014-1737-9. Epub 2014 Jun 19. J Cancer Res Clin Oncol. 2014. PMID: 24942335 Free PMC article.
Cited by
-
Once-Weekly 1.6 mg/m2 Bortezomib BCD Regimen in Elderly Patients with Newly Diagnosed Multiple Myeloma Who are Unfit for Standard Dose Chemotherapy.Indian J Hematol Blood Transfus. 2017 Mar;33(1):22-30. doi: 10.1007/s12288-016-0647-1. Epub 2016 Jan 27. Indian J Hematol Blood Transfus. 2017. PMID: 28194052 Free PMC article.
-
Bortezomib, lenalidomide, and dexamethasone versus bortezomib, doxorubicin, and dexamethasone in newly diagnosed multiple myeloma.BMC Cancer. 2024 Sep 9;24(1):1123. doi: 10.1186/s12885-024-12880-9. BMC Cancer. 2024. PMID: 39251979 Free PMC article.
-
Bortezomib, epirubicin, and dexamethasone (PAD) results in superior free-progression survival compared to bortezomib, cyclophosphamide, and dexamethasone (VCD) treatment in non-transplantation newly diagnosed multiple myeloma patients aged between 50 to 65: a retrospective single-center analysis in non-transplant patients.Ann Transl Med. 2022 Jun;10(12):674. doi: 10.21037/atm-22-394. Ann Transl Med. 2022. PMID: 35845500 Free PMC article.
-
How to determine post-FCR therapy for cytogenetic risk-tailored elderly patients with chronic lymphocytic leukemia, maintenance rituximab or observation.Med Oncol. 2014 Aug;31(8):104. doi: 10.1007/s12032-014-0104-7. Epub 2014 Jul 2. Med Oncol. 2014. PMID: 24985882 Clinical Trial.
-
A systematic review on the epidemiology and treatment options of multiple Myeloma in Asia.Heliyon. 2024 Oct 22;10(21):e39698. doi: 10.1016/j.heliyon.2024.e39698. eCollection 2024 Nov 15. Heliyon. 2024. PMID: 39553611 Free PMC article.
References
-
- Anagnostopoulos A, Gika D, Symeonidis A, Zervas K, Pouli A, Repoussis P et al (2005) Multiple myeloma in elderly patients: prognostic factors and outcome. Eur J Haematol 75:370–375 - PubMed
-
- Bladé J, Samson D, Reece D, Apperley J, Björkstrand B, Gahrton G et al (1998) Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma subcommittee of the EBMT. European group for blood and marrow transplant. Br J Haematol 102:1115–1123 - PubMed
-
- Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Bladé J et al (2005) International staging system for multiple myeloma. J Clin Oncol 23:3412–3420 - PubMed
-
- Harousseau JL, Attal M, Avet-Loiseau H, Marit G, Caillot D, Mohty M et al (2010) Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: results of the IFM 2005-01 phase III trial. J Clin Oncol 28:4621–4629 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical