Stewardship practices of U.S. biobanks
- PMID: 24337477
- PMCID: PMC4185188
- DOI: 10.1126/scitranslmed.3007362
Stewardship practices of U.S. biobanks
Abstract
Biobanks require new governance models that address their ethical and regulatory challenges. One model relies on stewardship of specimens throughout their life course. Here, we discuss findings from our survey of 456 U.S. biobank managers that addressed whether and how biobanks steward their specimens. The findings reveal that most biobanks do not create ongoing relationships with contributors but do practice stewardship over storing and sharing of specimens. Biobanks now need guidance to fully articulate stewardship practices that ensure respect for contributors while facilitating research.
Figures
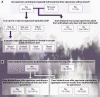

References
-
- Department of Health and Human Services Code of Federal Regulations Title 45, Part 46: Protection of human subjects. 2009 www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html.
-
- Henderson GE. Is informed consent broken? Am. J. Med. Sci. 2011;342:267–272. - PubMed
-
- Wolf SM, Crock BN, Van Ness B, Lawrenz F, Kahn JP, Beskow LM, Cho MK, Christman MF, Green RC, Hall R, Illes J, Keane M, Knoppers BM, Koenig BA, Kohane IS, Leroy B, Maschke KJ, McGeveran W, Ossorio P, Parker LS, Petersen GM, Richardson HS, Scott JA, Terry SF, Wilfond BS, Wolf WA. Managing incidental findings and research results in genomic research involving biobanks and archived data sets. Genet. Med. 2012;14:361–384. - PMC - PubMed
-
- Collins FS, Green ED, Guttmacher AE, Guyer MS. A vision for the future of genomics research. Nature. 2003;422:835–847. - PubMed
-
- Green ED, Guyer MS. National Human Genome Research Institute, Charting a course for genomic medicine from base pairs to bedside. Nature. 2011;470:204–213. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

