PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses
- PMID: 24337479
- PMCID: PMC4238416
- DOI: 10.1126/scitranslmed.3006597
PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses
Abstract
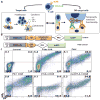
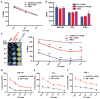
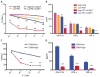
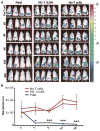
T cell therapies have demonstrated long-term efficacy and curative potential for the treatment of some cancers. However, their use is limited by damage to bystander tissues, as seen in graft-versus-host disease after donor lymphocyte infusion, or "on-target, off-tumor" toxicities incurred in some engineered T cell therapies. Nonspecific immunosuppression and irreversible T cell elimination are currently the only means to control such deleterious responses, but at the cost of abrogating therapeutic benefits or causing secondary complications. On the basis of the physiological paradigm of immune inhibitory receptors, we designed antigen-specific inhibitory chimeric antigen receptors (iCARs) to preemptively constrain T cell responses. We demonstrate that CTLA-4- or PD-1-based iCARs can selectively limit cytokine secretion, cytotoxicity, and proliferation induced through the endogenous T cell receptor or an activating chimeric receptor. The initial effect of the iCAR is temporary, thus enabling T cells to function upon a subsequent encounter with the antigen recognized by their activating receptor. iCARs thus provide a dynamic, self-regulating safety switch to prevent, rather than treat, the consequences of inadequate T cell specificity.
Conflict of interest statement
Figures



References
-
- Blattman JN, Greenberg PD. Cancer immunotherapy: A treatment for the masses. Science. 2004;305:200–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

