A role for peroxisome proliferator-activated receptor γ coactivator 1 (PGC-1) in the regulation of cardiac mitochondrial phospholipid biosynthesis
- PMID: 24337569
- PMCID: PMC3900969
- DOI: 10.1074/jbc.M113.523654
A role for peroxisome proliferator-activated receptor γ coactivator 1 (PGC-1) in the regulation of cardiac mitochondrial phospholipid biosynthesis
Abstract
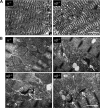
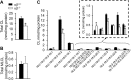
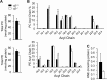
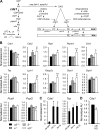
The energy demands of the adult mammalian heart are met largely by ATP generated via oxidation of fatty acids in a high capacity mitochondrial system. Peroxisome proliferator-activated receptor γ coactivator 1 (PGC-1)-α and -β serve as inducible transcriptional coregulators of genes involved in mitochondrial biogenesis and metabolism. Whether PGC-1 plays a role in the regulation of mitochondrial structure is unknown. In this study, mice with combined deficiency of PGC-1α and PGC-1β (PGC-1αβ(-/-)) in adult heart were analyzed. PGC-1αβ(-/-) hearts exhibited a distinctive mitochondrial cristae-stacking abnormality suggestive of a phospholipid abnormality as has been described in humans with genetic defects in cardiolipin (CL) synthesis (Barth syndrome). A subset of molecular species, containing n-3 polyunsaturated species in the CL, phosphatidylcholine, and phosphatidylethanolamine profiles, was reduced in PGC-1αβ-deficient hearts. Gene expression profiling of PGC-1αβ(-/-) hearts revealed reduced expression of the gene encoding CDP-diacylglycerol synthase 1 (Cds1), an enzyme that catalyzes the proximal step in CL biosynthesis. Cds1 gene promoter-reporter cotransfection experiments and chromatin immunoprecipitation studies demonstrated that PGC-1α coregulates estrogen-related receptors to activate the transcription of the Cds1 gene. We conclude that the PGC-1/estrogen-related receptor axis coordinately regulates metabolic and membrane structural programs relevant to the maintenance of high capacity mitochondrial function in heart.
Keywords: Cardiac Metabolism; Cardiolipin; Gene Regulation; Heart; Lipidomics; Mitochondria; Phospholipids; Polyunsaturated Fatty Acids; Transcriptional Coregulators.
Figures
References
-
- Zambrano F., Fleischer S., Fleischer B. (1975) Lipid composition of the Golgi apparatus of rat kidney and liver in comparison with other subcellular organelles. Biochim. Biophys. Acta 380, 357–369 - PubMed
-
- Bissler J. J., Tsoras M., Göring H. H., Hug P., Chuck G., Tombragel E., McGraw C., Schlotman J., Ralston M. A., Hug G. (2002) Infantile dilated X-linked cardiomyopathy, G4.5 mutations, altered lipids, and ultrastructural malformations of mitochondria in heart, liver, and skeletal muscle. Lab. Invest. 82, 335–344 - PubMed
-
- Hallman M. (1971) Changes in mitochondrial respiratory chain proteins during perinatal development. Evidence of the importance of environmental oxygen tension. Biochim. Biophys. Acta 253, 360–372 - PubMed
-
- Smolich J. J., Walker A. M., Campbell G. R., Adamson T. M. (1989) Left and right ventricular myocardial morphometry in fetal, neonatal, and adult sheep. Am. J. Physiol. 257, H1–H9 - PubMed
-
- Marin-Garcia J., Ananthakrishnan R., Goldenthal M. J. (2000) Heart mitochondrial DNA and enzyme changes during early human development. Mol. Cell. Biochem. 210, 47–52 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

