Amino acids activate mammalian target of rapamycin (mTOR) complex 1 without changing Rag GTPase guanyl nucleotide charging
- PMID: 24337580
- PMCID: PMC3908400
- DOI: 10.1074/jbc.M113.528505
Amino acids activate mammalian target of rapamycin (mTOR) complex 1 without changing Rag GTPase guanyl nucleotide charging
Abstract
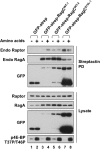
Activation of mammalian target of rapamycin complex 1 (mTORC1) by amino acids is mediated in part by the Rag GTPases, which bind the raptor subunit of mTORC1 in an amino acid-stimulated manner and promote mTORC1 interaction with Rheb-GTP, the immediate activator. Here we examine whether the ability of amino acids to regulate mTORC1 binding to Rag and mTORC1 activation is due to the regulation of Rag guanyl nucleotide charging. Rag heterodimers in vitro exhibit a very rapid, spontaneous exchange of guanyl nucleotides and an inability to hydrolyze GTP. Mutation of the Rag P-loop corresponding to Ras(Ser-17) abolishes guanyl nucleotide binding. Such a mutation in RagA or RagB inhibits, whereas in RagC or RagD it enhances, Rag heterodimer binding to mTORC1. The binding of wild-type and mutant Rag heterodimers to mTORC1 in vitro parallels that seen with transient expression, but binding to mTORC1 in vitro is entirely independent of Rag guanyl nucleotide charging. HeLa cells stably overexpressing wild-type or P-loop mutant RagC exhibit unaltered amino acid regulation of mTORC1. Despite amino acid-independent raptor binding to Rag, mTORC1 is inhibited by amino acid withdrawal as in parental cells. Rag heterodimers extracted from (32)P-labeled whole cells, or just from the pool associated with the lysosomal membrane, exhibit constitutive [(32)P]GTP charging that is unaltered by amino acid withdrawal. Thus, amino acids promote mTORC1 activation without altering Rag GTP charging. Raptor binding to Rag, although necessary, is not sufficient for mTORC1 activation. Additional amino acid-dependent steps couple Rag-mTORC1 to Rheb-GTP.
Keywords: Amino Acids; Cell Signaling; GTPase; Insulin; Lysosomes; Rag GTPase; Raptor; mTOR Complex (mTORC).
Figures





Similar articles
-
Amino acid regulation of TOR complex 1.Am J Physiol Endocrinol Metab. 2009 Apr;296(4):E592-602. doi: 10.1152/ajpendo.90645.2008. Epub 2008 Sep 2. Am J Physiol Endocrinol Metab. 2009. PMID: 18765678 Free PMC article. Review.
-
Amino acid-dependent NPRL2 interaction with Raptor determines mTOR Complex 1 activation.Cell Signal. 2016 Feb;28(2):32-41. doi: 10.1016/j.cellsig.2015.11.008. Epub 2015 Nov 12. Cell Signal. 2016. PMID: 26582740
-
Rag GTPase in amino acid signaling.Amino Acids. 2016 Apr;48(4):915-928. doi: 10.1007/s00726-016-2171-x. Epub 2016 Jan 18. Amino Acids. 2016. PMID: 26781224 Review.
-
The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1.Science. 2008 Jun 13;320(5882):1496-501. doi: 10.1126/science.1157535. Epub 2008 May 22. Science. 2008. PMID: 18497260 Free PMC article.
-
Amino Acid-Dependent mTORC1 Regulation by the Lysosomal Membrane Protein SLC38A9.Mol Cell Biol. 2015 Jul;35(14):2479-94. doi: 10.1128/MCB.00125-15. Epub 2015 May 11. Mol Cell Biol. 2015. PMID: 25963655 Free PMC article.
Cited by
-
Milk: an epigenetic amplifier of FTO-mediated transcription? Implications for Western diseases.J Transl Med. 2015 Dec 21;13:385. doi: 10.1186/s12967-015-0746-z. J Transl Med. 2015. PMID: 26691922 Free PMC article. Review.
-
Positive and Negative Regulation of the Master Metabolic Regulator mTORC1 by Two Families of Legionella pneumophila Effectors.Cell Rep. 2017 Nov 21;21(8):2031-2038. doi: 10.1016/j.celrep.2017.10.088. Cell Rep. 2017. PMID: 29166595 Free PMC article.
-
Tumor-derived microparticles in tumor immunology and immunotherapy.Eur J Immunol. 2020 Nov;50(11):1653-1662. doi: 10.1002/eji.202048548. Epub 2020 Oct 28. Eur J Immunol. 2020. PMID: 32976623 Free PMC article. Review.
-
Human T2D-Associated Gene IMP2/IGF2BP2 Promotes the Commitment of Mesenchymal Stem Cells Into Adipogenic Lineage.Diabetes. 2023 Jan 1;72(1):33-44. doi: 10.2337/db21-1087. Diabetes. 2023. PMID: 36219823 Free PMC article.
-
Recurrent Mutations in the MTOR Regulator RRAGC in Follicular Lymphoma.Clin Cancer Res. 2016 Nov 1;22(21):5383-5393. doi: 10.1158/1078-0432.CCR-16-0609. Epub 2016 Jun 7. Clin Cancer Res. 2016. PMID: 27267853 Free PMC article.
References
-
- Hara K., Maruki Y., Long X., Yoshino K., Oshiro N., Hidayat S., Tokunaga C., Avruch J., Yonezawa K. (2002) Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110, 177–189 - PubMed
-
- Kim D. H., Sarbassov D. D., Ali S. M., King J. E., Latek R. R., Erdjument-Bromage H., Tempst P., Sabatini D. M. (2002) mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110, 163–175 - PubMed
-
- Loewith R., Jacinto E., Wullschleger S., Lorberg A., Crespo J. L., Bonenfant D., Oppliger W., Jenoe P., Hall M. N. (2002) Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol. Cell 10, 457–468 - PubMed
-
- Long X., Lin Y., Ortiz-Vega S., Yonezawa K., Avruch J. (2005) Rheb binds and regulates the mTOR kinase. Curr. Biol. 15, 702–713 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

