Phase 1 study of efatutazone, a novel oral peroxisome proliferator-activated receptor gamma agonist, in combination with FOLFIRI as second-line therapy in patients with metastatic colorectal cancer
- PMID: 24337768
- PMCID: PMC4045340
- DOI: 10.1007/s10637-013-0056-3
Phase 1 study of efatutazone, a novel oral peroxisome proliferator-activated receptor gamma agonist, in combination with FOLFIRI as second-line therapy in patients with metastatic colorectal cancer
Abstract
Background: Efatutazone, a novel oral highly-selective peroxisome proliferator-activated receptor gamma (PPARγ) agonist, has demonstrated some inhibitory effects on disease stabilization in patients with metastatic colorectal cancer (mCRC) enrolled in previous phase I studies. Here, we evaluate the safety and pharmacokinetics of efatutazone combined with FOLFIRI (5-fluorouracil, levo-leucovorin, and irinotecan) as second-line chemotherapy in Japanese patients with mCRC.
Methods: Dose-limiting toxicities (DLTs) were evaluated at 2 efatutazone dose levels of 0.25 and 0.50 mg (the recommended dose [RD] of efatutazone monotherapy) twice daily in combination with FOLFIRI in a 3-9 patient cohort. Furthermore, tolerability at the RD level was assessed in additional patients, up to 12 in total. Blood samples for pharmacokinetics and biomarkers and tumor samples for archival tissues were collected from all patients.
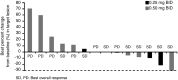
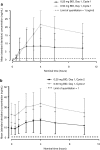
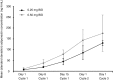
Results: Fifteen patients (0.25 mg, 3; 0.5 mg, 12) were enrolled. No DLTs were observed. Most patients experienced weight increase (100 %) and edema (80.0 %), which were manageable with diuretics. Common grade 3/4 toxicities were neutropenia (93.3 %), leukopenia (46.7 %), and anemia (33.3 %). Stable disease was observed in 8 of the 14 patients evaluable for tumor response. The plasma adiponectin levels increased over time and increased dose. No clear relationship was detected between treatment efficacies and plasma levels of adiponectin as well as the expression levels of PPARγ and the retinoid X receptor in tumor tissues.
Conclusions: Efatutazone combined with FOLFIRI demonstrates an acceptable safety profile and evidence of disease stabilization in Japanese patients with mCRC. The RD for efatutazone monotherapy can be used in combination with FOLFIRI.
Figures
Similar articles
-
A phase 1 study of efatutazone, an oral peroxisome proliferator-activated receptor gamma agonist, administered to patients with advanced malignancies.Cancer. 2012 Nov 1;118(21):5403-13. doi: 10.1002/cncr.27526. Epub 2012 May 8. Cancer. 2012. PMID: 22570147 Free PMC article. Clinical Trial.
-
A phase I study of intravenous aflibercept with FOLFIRI in Japanese patients with previously treated metastatic colorectal cancer.Invest New Drugs. 2013 Aug;31(4):910-7. doi: 10.1007/s10637-012-9895-6. Epub 2012 Nov 20. Invest New Drugs. 2013. PMID: 23179335 Free PMC article. Clinical Trial.
-
Phase I pharmacokinetic/pharmacodynamic study of EKB-569, an irreversible inhibitor of the epidermal growth factor receptor tyrosine kinase, in combination with irinotecan, 5-fluorouracil, and leucovorin (FOLFIRI) in first-line treatment of patients with metastatic colorectal cancer.Clin Cancer Res. 2008 Jan 1;14(1):215-23. doi: 10.1158/1078-0432.CCR-07-1053. Clin Cancer Res. 2008. PMID: 18172273 Clinical Trial.
-
A phase I study of sunitinib in combination with FOLFIRI in patients with untreated metastatic colorectal cancer.Ann Oncol. 2012 Jan;23(1):119-127. doi: 10.1093/annonc/mdr046. Epub 2011 Mar 29. Ann Oncol. 2012. PMID: 21447616 Clinical Trial.
-
Phase I trial of irinotecan, infusional 5-fluorouracil, and leucovorin (FOLFIRI) with erlotinib (OSI-774): early termination due to increased toxicities.Clin Cancer Res. 2004 Oct 1;10(19):6522-7. doi: 10.1158/1078-0432.CCR-04-0746. Clin Cancer Res. 2004. PMID: 15475439 Clinical Trial.
Cited by
-
Peroxisome proliferator-activated receptor-γ agonist troglitazone suppresses transforming growth factor-β1 signalling through miR-92b upregulation-inhibited Axl expression in human keloid fibroblasts in vitro.Am J Transl Res. 2016 Aug 15;8(8):3460-70. eCollection 2016. Am J Transl Res. 2016. PMID: 27648136 Free PMC article.
-
Delineating the role of nuclear receptors in colorectal cancer, a focused review.Discov Oncol. 2024 Feb 19;15(1):41. doi: 10.1007/s12672-023-00808-x. Discov Oncol. 2024. PMID: 38372868 Free PMC article. Review.
-
Natural compounds targeting nuclear receptors for effective cancer therapy.Cancer Metastasis Rev. 2023 Sep;42(3):765-822. doi: 10.1007/s10555-022-10068-w. Epub 2022 Dec 8. Cancer Metastasis Rev. 2023. PMID: 36482154 Review.
-
Vetting of New 2,5-Bis (2,2,2-trifluoroethoxy) Phenyl-Linked 1,3-Thiazolidine-4-one Derivatives as AURKA and VEGFR-2 Inhibitor Antiglioma Agents Assisted with In Vitro and In Silico Studies.ACS Omega. 2023 Nov 10;8(46):43596-43609. doi: 10.1021/acsomega.3c04662. eCollection 2023 Nov 21. ACS Omega. 2023. PMID: 38027362 Free PMC article.
-
Troglitazone Impedes the Oligomerization of Sodium Taurocholate Cotransporting Polypeptide and Entry of Hepatitis B Virus Into Hepatocytes.Front Microbiol. 2019 Jan 8;9:3257. doi: 10.3389/fmicb.2018.03257. eCollection 2018. Front Microbiol. 2019. PMID: 30671048 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

