Redox regulation of apurinic/apyrimidinic endonuclease 1 activity in Long-Evans Cinnamon rats during spontaneous hepatitis
- PMID: 24337968
- PMCID: PMC4218752
- DOI: 10.1007/s11010-013-1909-y
Redox regulation of apurinic/apyrimidinic endonuclease 1 activity in Long-Evans Cinnamon rats during spontaneous hepatitis
Abstract
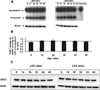
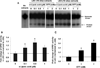
The Long-Evans Cinnamon (LEC) rat is an animal model for Wilson's disease. This animal is genetically predisposed to copper accumulation in the liver, increased oxidative stress, accumulation of DNA damage, and the spontaneous development of hepatocellular carcinoma. Thus, this animal model is useful for studying the relationship of endogenous DNA damage to spontaneous carcinogenesis. In this study, we have investigated the apurinic/apyrimidinic endonuclease 1 (APE1)-mediated excision repair of endogenous DNA damage, apurinic/apyrimidinic (AP)-sites, which is highly mutagenic and implicated in human cancer. We found that the activity was reduced in the liver extracts from the acute hepatitis period of LEC rats as compared with extracts from the age-matched Long-Evans Agouti rats. The acute hepatitis period had also a heightened oxidative stress condition as assessed by an increase in oxidized glutathione level and loss of enzyme activity of glyceraldehyde 3-phosphate dehydrogenase, a key redox-sensitive protein in cells. Interestingly, the activity reduction was not due to changes in protein expression but apparently by reversible protein oxidation as the addition of reducing agents to extracts of the liver from acute hepatitis period reactivated APE1 activity and thus, confirmed the oxidation-mediated loss of APE1 activity under increased oxidative stress. These findings show for the first time in an animal model that the repair mechanism of AP-sites is impaired by increased oxidative stress in acute hepatitis via redox regulation which contributed to the increased accumulation of mutagenic AP-sites in liver DNA.
Conflict of interest statement
Figures



Similar articles
-
Evidence of alterations in base excision repair of oxidative DNA damage during spontaneous hepatocarcinogenesis in Long Evans Cinnamon rats.Cancer Res. 2003 Nov 15;63(22):7704-7. Cancer Res. 2003. PMID: 14633694
-
Suppression of tumor suppressor Tsc2 and DNA repair glycosylase Nth1 during spontaneous liver tumorigenesis in Long-Evans Cinnamon rats.Mol Cell Biochem. 2010 May;338(1-2):233-9. doi: 10.1007/s11010-009-0357-1. Epub 2009 Dec 24. Mol Cell Biochem. 2010. PMID: 20033472 Free PMC article.
-
Methods to Assess Oxidative DNA Base Damage Repair of Apurinic/Apyrimidinic (AP) Sites Using Radioactive and Nonradioactive Oligonucleotide-Based Assays.Methods Mol Biol. 2022;2413:155-163. doi: 10.1007/978-1-0716-1896-7_16. Methods Mol Biol. 2022. PMID: 35044663
-
AP Endonuclease 1 as a Key Enzyme in Repair of Apurinic/Apyrimidinic Sites.Biochemistry (Mosc). 2016 Sep;81(9):951-67. doi: 10.1134/S0006297916090042. Biochemistry (Mosc). 2016. PMID: 27682167 Review.
-
Inhibitors of nuclease and redox activity of apurinic/apyrimidinic endonuclease 1/redox effector factor 1 (APE1/Ref-1).Bioorg Med Chem. 2017 May 1;25(9):2531-2544. doi: 10.1016/j.bmc.2017.01.028. Epub 2017 Jan 21. Bioorg Med Chem. 2017. PMID: 28161249 Review.
Cited by
-
Lead facilitates foci formation in a Balb/c-3T3 two-step cell transformation model: role of Ape1 function.Environ Sci Pollut Res Int. 2018 Apr;25(12):12150-12158. doi: 10.1007/s11356-018-1396-5. Epub 2018 Feb 17. Environ Sci Pollut Res Int. 2018. PMID: 29455351
References
-
- Mangerich A, Knutson CG, Parry NM, Muthupalani S, Ye W, Prestwich E, Cui L, McFaline JL, Mobley M, Ge Z, Taghizadeh K, Wishnok JS, Wogan GN, Fox JG, Tannenbaum SR, Dedon PC. Infection-induced colitis in mice causes dynamic and tissue-specific changes in stress response and DNA damage leading to colon cancer. Proc Natl Acad Sci U S A. 2012;109:1820–1829. - PMC - PubMed
-
- Jena G, Trivedi PP, Sandala B. Oxidative stress in ulcerative colitis: an old concept but a new concern. Free Radic Res. 2012;46:1339–1345. - PubMed
-
- de Moura MB, dos Santos LS, Van Houten B. Mitochondrial dysfunction in neurodegenerative diseases and cancer. Environ Mol Mutagen. 2010;51:391–405. - PubMed
-
- Hussain SP, Raja K, Amstad PA, Sawyer M, Trudel LJ, Wogan GN, Hofseth LJ, Shields PG, Billiar TR, Trautwein C, Hohler T, Galle PR, Phillips DH, Markin R, Marrogi AJ, Harris CC. Increased p53 mutation load in nontumorous human liver of Wilson disease and hemochromatosis: Oxyradical overload diseases. Proc Nat Acad Sci U S A. 2000;97:12770–12775. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

