The interaction of integrin αIIbβ3 with fibrin occurs through multiple binding sites in the αIIb β-propeller domain
- PMID: 24338009
- PMCID: PMC3900980
- DOI: 10.1074/jbc.M113.518126
The interaction of integrin αIIbβ3 with fibrin occurs through multiple binding sites in the αIIb β-propeller domain
Abstract
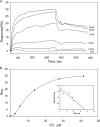
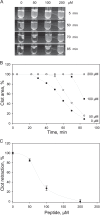
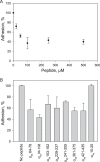
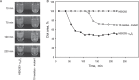
The currently available antithrombotic agents target the interaction of platelet integrin αIIbβ3 (GPIIb-IIIa) with fibrinogen during platelet aggregation. Platelets also bind fibrin formed early during thrombus growth. It was proposed that inhibition of platelet-fibrin interactions may be a necessary and important property of αIIbβ3 antagonists; however, the mechanisms by which αIIbβ3 binds fibrin are uncertain. We have previously identified the γ370-381 sequence (P3) in the γC domain of fibrinogen as the fibrin-specific binding site for αIIbβ3 involved in platelet adhesion and platelet-mediated fibrin clot retraction. In the present study, we have demonstrated that P3 can bind to several discontinuous segments within the αIIb β-propeller domain of αIIbβ3 enriched with negatively charged and aromatic residues. By screening peptide libraries spanning the sequence of the αIIb β-propeller, several sequences were identified as candidate contact sites for P3. Synthetic peptides duplicating these segments inhibited platelet adhesion and clot retraction but not platelet aggregation, supporting the role of these regions in fibrin recognition. Mutant αIIbβ3 receptors in which residues identified as critical for P3 binding were substituted for homologous residues in the I-less integrin αMβ2 exhibited reduced cell adhesion and clot retraction. These residues are different from those that are involved in the coordination of the fibrinogen γ404-411 sequence and from auxiliary sites implicated in binding of soluble fibrinogen. These results map the binding of fibrin to multiple sites in the αIIb β-propeller and further indicate that recognition specificity of αIIbβ3 for fibrin differs from that for soluble fibrinogen.
Keywords: Adhesion; Clot Retraction; Fibrin; Fibrinogen; Integrin; Platelets.
Figures



Similar articles
-
[Structure-functional analysis of peptide P3 identical to gamma370-383 binding sites with integrin alphaIIbbeta3 in gammaC-domain of fibrinogen].Ukr Biokhim Zh (1999). 2003 Nov-Dec;75(6):99-105. Ukr Biokhim Zh (1999). 2003. PMID: 15143525 Ukrainian.
-
A cluster of basic amino acid residues in the gamma370-381 sequence of fibrinogen comprises a binding site for platelet integrin alpha(IIb)beta3 (glycoprotein IIb/IIIa).Biochemistry. 2005 Dec 27;44(51):16920-30. doi: 10.1021/bi051581d. Biochemistry. 2005. PMID: 16363805
-
Identification of a novel binding site for platelet integrins alpha IIb beta 3 (GPIIbIIIa) and alpha 5 beta 1 in the gamma C-domain of fibrinogen.J Biol Chem. 2003 Aug 22;278(34):32251-8. doi: 10.1074/jbc.M300410200. Epub 2003 Jun 10. J Biol Chem. 2003. PMID: 12799374
-
Recent development of peptides from glycoproteins IIb (alphaIIb) and IIIa (beta3) that inhibit platelet fibrinogen binding.Curr Med Chem Cardiovasc Hematol Agents. 2005 Apr;3(2):99-103. doi: 10.2174/1568016053544345. Curr Med Chem Cardiovasc Hematol Agents. 2005. PMID: 15853697 Review.
-
Regulation of integrins in platelets.Biopolymers. 2015 Jul;104(4):323-33. doi: 10.1002/bip.22679. Biopolymers. 2015. PMID: 26010651 Review.
Cited by
-
Congenital hypodysfibrinogenemia associated with a novel deletion of three residues (γAla289_Asp291del) in fibrinogen.J Thromb Thrombolysis. 2018 Aug;46(2):211-218. doi: 10.1007/s11239-018-1678-2. J Thromb Thrombolysis. 2018. PMID: 29748775
-
Platelets Promote Thromboinflammation in SARS-CoV-2 Pneumonia.Arterioscler Thromb Vasc Biol. 2020 Dec;40(12):2975-2989. doi: 10.1161/ATVBAHA.120.315175. Epub 2020 Oct 14. Arterioscler Thromb Vasc Biol. 2020. PMID: 33052054 Free PMC article.
-
Novel Role for the AnxA1-Fpr2/ALX Signaling Axis as a Key Regulator of Platelet Function to Promote Resolution of Inflammation.Circulation. 2019 Jul 23;140(4):319-335. doi: 10.1161/CIRCULATIONAHA.118.039345. Epub 2019 Jun 3. Circulation. 2019. PMID: 31154815 Free PMC article.
-
Experimental and Mathematical Model of Platelet Hemostasis Kinetics.Cells. 2025 May 7;14(9):677. doi: 10.3390/cells14090677. Cells. 2025. PMID: 40358201 Free PMC article.
-
The Interplay between Non-Esterified Fatty Acids and Plasma Zinc and Its Influence on Thrombotic Risk in Obesity and Type 2 Diabetes.Int J Mol Sci. 2021 Sep 20;22(18):10140. doi: 10.3390/ijms221810140. Int J Mol Sci. 2021. PMID: 34576303 Free PMC article. Review.
References
-
- Falati S., Gross P., Merrill-Skoloff G., Furie B. C., Furie B. (2002) Real-time in vivo imaging of platelets, tissue factor and fibrin during arterial thrombus formation in the mouse. Nat. Med. 8, 1175–1181 - PubMed
-
- Hayashi T., Mogami H., Murakami Y., Nakamura T., Kanayama N., Konno H., Urano T. (2008) Real-time analysis of platelet aggregation and procoagulant activity during thrombus formation in vivo. Pflugers Arch. 456, 1239–1251 - PubMed
-
- Hechler B., Nonne C., Eckly A., Magnenat S., Rinckel J. Y., Denis C. V., Freund M., Cazenave J. P., Lanza F., Gachet C. (2010) Arterial thrombosis: relevance of a model with two levels of severity assessed by histologic, ultrastructural and functional characterization. J. Thromb. Haemost. 8, 173–184 - PubMed
-
- Nurden A. T., Nurden P. (2003) GPIIb/IIIa Antagonists and other anti-integrins. Semin. Vasc. Med. 3, 123–130 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

