N-terminal acetylation stabilizes N-terminal helicity in lipid- and micelle-bound α-synuclein and increases its affinity for physiological membranes
- PMID: 24338013
- PMCID: PMC3916564
- DOI: 10.1074/jbc.M113.512459
N-terminal acetylation stabilizes N-terminal helicity in lipid- and micelle-bound α-synuclein and increases its affinity for physiological membranes
Abstract
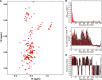
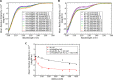
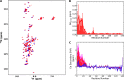
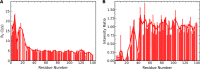
The Parkinson disease protein α-synuclein is N-terminally acetylated, but most in vitro studies have been performed using unacetylated α-synuclein. Binding to lipid membranes is considered key to the still poorly understood function of α-synuclein. We report the effects of N-terminal acetylation on α-synuclein binding to lipid vesicles of different composition and curvature and to micelles composed of the detergents β-octyl-glucoside (BOG) and SDS. In the presence of SDS, N-terminal acetylation results in a slightly increased helicity for the N-terminal ~10 residues of the protein, likely due to the stabilization of N-terminal fraying through the formation of a helix cap motif. In the presence of BOG, a detergent used in previous isolations of helical oligomeric forms of α-synuclein, the N-terminally acetylated protein adopts a novel conformation in which the N-terminal ~30 residues bind the detergent micelle in a partly helical conformation, whereas the remainder of the protein remains unbound and disordered. Binding of α-synuclein to lipid vesicles with high negative charge content is essentially unaffected by N-terminal acetylation irrespective of curvature, but binding to vesicles of lower negative charge content is increased, with stronger binding observed for vesicles with higher curvature. Thus, the naturally occurring N-terminally acetylated form of α-synuclein exhibits stabilized helicity at its N terminus and increased affinity for lipid vesicles similar to synaptic vesicles, a binding target of the protein in vivo. Furthermore, the novel BOG-bound state of N-terminally acetylated α-synuclein may serve as a model of partly helical membrane-bound intermediates with a role in α-synuclein function and dysfunction.
Keywords: Lipid-binding Protein; N-terminal Acetylation; Parkinson Disease; Post Translational Modification; Protein Aggregation; Synuclein; α-Synuclein.
Figures



References
-
- Arnesen T., Van Damme P., Polevoda B., Helsens K., Evjenth R., Colaert N., Varhaug J. E., Vandekerckhove J., Lillehaug J. R., Sherman F., Gevaert K. (2009) Proteomics analyses reveal the evolutionary conservation and divergence of N-terminal acetyltransferases from yeast and humans. Proc. Natl. Acad. Sci. U.S.A. 106, 8157–8162 - PMC - PubMed
-
- Jarvis J. A., Ryan M. T., Hoogenraad N. J., Craik D. J., Høj P. B. (1995) Solution structure of the acetylated and noncleavable mitochondrial targeting signal of rat chaperonin 10. J. Biol. Chem. 270, 1323–1331 - PubMed
-
- Kimura Y., Takaoka M., Tanaka S., Sassa H., Tanaka K., Polevoda B., Sherman F., Hirano H. (2000) nα-α-Acetylation and proteolytic activity of the yeast 20 S proteasome. J. Biol. Chem. 275, 4635–4639 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

