Nuclear import factor Srp1 and its associated protein Sts1 couple ribosome-bound nascent polypeptides to proteasomes for cotranslational degradation
- PMID: 24338021
- PMCID: PMC3908403
- DOI: 10.1074/jbc.M113.524926
Nuclear import factor Srp1 and its associated protein Sts1 couple ribosome-bound nascent polypeptides to proteasomes for cotranslational degradation
Abstract
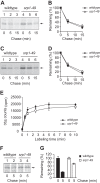
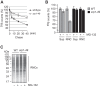
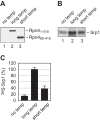
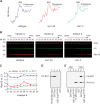
Cotranslational protein degradation plays an important role in protein quality control and proteostasis. Although ubiquitylation has been suggested to signal cotranslational degradation of nascent polypeptides, cotranslational ubiquitylation occurs at a low level, suggesting the existence of an alternative route for delivery of nascent polypeptides to the proteasome. Here we report that the nuclear import factor Srp1 (also known as importin α or karyopherin α) is required for ubiquitin-independent cotranslational degradation of the transcription factor Rpn4. We further demonstrate that cotranslational protein degradation is generally impaired in the srp1-49 mutant. Srp1 binds nascent polypeptides emerging from the ribosome. The association of proteasomes with polysomes is weakened in srp1-49. The interaction between Srp1 and the proteasome is mediated by Sts1, a multicopy suppressor of srp1-49. The srp1-49 and sts1-2 mutants are hypersensitive to stressors that promote protein misfolding, underscoring the physiological function of Srp1 and Sts1 in degradation of misfolded nascent polypeptides. This study unveils a previously unknown role for Srp1 and Sts1 in cotranslational protein degradation and suggests a novel model whereby Srp1 and Sts1 cooperate to couple proteasomes to ribosome-bound nascent polypeptides.
Keywords: Cotranslational Protein Degradation; Nuclear Import Factor Srp1; Proteasome; Protein Degradation; Protein Dynamics; Protein Processing; Protein Turnover; Ubiquitin-independent Degradation.
Figures


References
-
- Hartl F. U., Bracher A., Hayer-Hartl M. (2011) Molecular chaperones in protein folding and proteostasis. Nature 475, 324–332 - PubMed
-
- Kramer G., Boehringer D., Ban N., Bukau B. (2009) The ribosome as a platform for co-translational processing, folding and targeting of newly synthesized proteins. Nat. Struct. Mol. Biol. 16, 589–597 - PubMed
-
- Preissler S., Deuerling E. (2012) Ribosome-associated chaperones as key players in proteostasis. Trends Biochem. Sci. 37, 274–283 - PubMed
-
- Tyedmers J., Mogk A., Bukau B. (2010) Cellular strategies for controlling protein aggregation. Nat. Rev. Mol. Cell Biol. 11, 777–788 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

