Signature amyloid β profiles are produced by different γ-secretase complexes
- PMID: 24338474
- PMCID: PMC3924297
- DOI: 10.1074/jbc.M113.530907
Signature amyloid β profiles are produced by different γ-secretase complexes
Abstract
γ-Secretase complexes are involved in the generation of amyloid-β (Aβ) in the brain. Therefore, γ-secretase has been proposed as a potential therapeutic target in Alzheimer disease (AD). Targeting γ-secretase activity in AD requires the pharmacological dissociation of the processing of physiological relevant substrates and the generation of "toxic" Aβ. Previous reports suggest the differential targeting of γ-secretase complexes, based on their subunit composition, as a valid strategy. However, little is known about the biochemical properties of the different complexes, and key questions regarding their Aβ product profiles should be first addressed. Here, we expressed, purified, and analyzed, under the same conditions, the endopeptidase and carboxypeptidase-like activities of the four γ-secretase complexes present in humans. We find that the nature of the catalytic subunit in the complex affects both activities. Interestingly, PSEN2 complexes discriminate between the Aβ40 and Aβ38 production lines, indicating that Aβ generation in one or the other pathway can be dissociated. In contrast, the APH1 subunit mainly affects the carboxypeptidase-like activity, with APH1B complexes favoring the generation of longer Aβ peptides. In addition, we determined that expression of a single human γ-secretase complex in cell lines retains the intrinsic attributes of the protease while present in the membrane, providing validation for the in vitro studies. In conclusion, our data show that each γ-secretase complex produces a characteristic Aβ signature. The qualitative and quantitative differences between different γ-secretase complexes could be used to advance drug development in AD and other disorders.
Keywords: Alzheimer Disease; Amyloid Beta; Amyloid Precursor Protein; Enzyme Mechanisms; Gamma-Secretase; Neurobiology; Secretases.
Figures

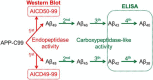

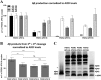
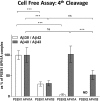

References
-
- Edbauer D., Winkler E., Regula J. T., Pesold B., Steiner H., Haass C. (2003) Reconstitution of γ-secretase activity. Nat. Cell Biol. 5, 486–488 - PubMed
-
- Tolia A., De Strooper B. (2009) Structure and function of γ-secretase. Semin. Cell Dev. Biol. 20, 211–218 - PubMed
-
- De Strooper B. (2003) Aph-1, Pen-2, and Nicastrin with Presenilin Generate an Active γ-Secretase Complex. Neuron 38, 9–12 - PubMed
-
- Gu Y., Chen F., Sanjo N., Kawarai T., Hasegawa H., Duthie M., Li W., Ruan X., Luthra A., Mount H. T., Tandon A., Fraser P. E., St George-Hyslop P. (2003) APH-1 interacts with mature and immature forms of presenilins and nicastrin and may play a role in maturation of presenilin.nicastrin complexes. J. Biol. Chem. 278, 7374–7380 - PubMed
-
- Vassar R., Bennett B. D., Babu-Khan S., Kahn S., Mendiaz E. A., Denis P., Teplow D. B., Ross S., Amarante P., Loeloff R., Luo Y., Fisher S., Fuller J., Edenson S., Lile J., Jarosinski M. A., Biere A. L., Curran E., Burgess T., Louis J. C., Collins F., Treanor J., Rogers G., Citron M. (1999) β-Secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286, 735–741 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

