Control of helicase loading in the coupled DNA replication and recombination systems of bacteriophage T4
- PMID: 24338568
- PMCID: PMC3908434
- DOI: 10.1074/jbc.M113.505842
Control of helicase loading in the coupled DNA replication and recombination systems of bacteriophage T4
Abstract
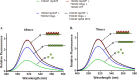
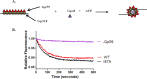
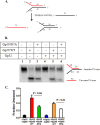
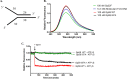
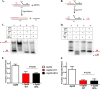
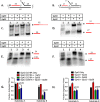
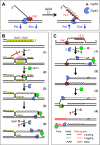
The Gp59 protein of bacteriophage T4 promotes DNA replication by loading the replicative helicase, Gp41, onto replication forks and recombination intermediates. Gp59 also blocks DNA synthesis by Gp43 polymerase until Gp41 is loaded, ensuring that synthesis is tightly coupled to unwinding. The distinct polymerase blocking and helicase loading activities of Gp59 likely involve different binding interactions with DNA and protein partners. Here, we investigate how interactions of Gp59 with DNA and Gp32, the T4 single-stranded DNA (ssDNA)-binding protein, are related to these activities. A previously characterized mutant, Gp59-I87A, exhibits markedly reduced affinity for ssDNA and pseudo-fork DNA substrates. We demonstrate that on Gp32-covered ssDNA, the DNA binding defect of Gp59-I87A is not detrimental to helicase loading and translocation. In contrast, on pseudo-fork DNA the I87A mutation is detrimental to helicase loading and unwinding in the presence or absence of Gp32. Other results indicate that Gp32 binding to lagging strand ssDNA relieves the blockage of Gp43 polymerase activity by Gp59, whereas the inhibition of Gp43 exonuclease activity is maintained. Our findings suggest that Gp59-Gp32 and Gp59-DNA interactions perform separate but complementary roles in T4 DNA metabolism; Gp59-Gp32 interactions are needed to load Gp41 onto D-loops, and other nucleoprotein structures containing clusters of Gp32. Gp59-DNA interactions are needed to load Gp41 onto nascent or collapsed replication forks lacking clusters of Gp32 and to coordinate bidirectional replication from T4 origins. The dual functionalities of Gp59 allow it to promote the initiation or re-start of DNA replication from a wide variety of recombination and replication intermediates.
Keywords: Bacteriophage; D-loop; DNA Helicase; DNA Repair; DNA Replication; Homologous Recombination; Mediator; Origin; R-loop; ssDNA-binding Protein.
Figures


Similar articles
-
Assembly and dynamics of Gp59-Gp32-single-stranded DNA (ssDNA), a DNA helicase loading complex required for recombination-dependent replication in bacteriophage T4.J Biol Chem. 2012 Jun 1;287(23):19070-81. doi: 10.1074/jbc.M112.343830. Epub 2012 Apr 12. J Biol Chem. 2012. PMID: 22500043 Free PMC article.
-
Helicase assembly protein Gp59 of bacteriophage T4: fluorescence anisotropy and sedimentation studies of complexes formed with derivatives of Gp32, the phage ssDNA binding protein.Biochemistry. 2001 Jun 26;40(25):7651-61. doi: 10.1021/bi010116n. Biochemistry. 2001. PMID: 11412119
-
Dual functions of single-stranded DNA-binding protein in helicase loading at the bacteriophage T4 DNA replication fork.J Biol Chem. 2004 Apr 30;279(18):19035-45. doi: 10.1074/jbc.M311738200. Epub 2004 Feb 9. J Biol Chem. 2004. PMID: 14871889
-
Mediator proteins orchestrate enzyme-ssDNA assembly during T4 recombination-dependent DNA replication and repair.Proc Natl Acad Sci U S A. 2001 Jul 17;98(15):8298-305. doi: 10.1073/pnas.131007498. Proc Natl Acad Sci U S A. 2001. PMID: 11459967 Free PMC article. Review.
-
Structural analysis of bacteriophage T4 DNA replication: a review in the Virology Journal series on bacteriophage T4 and its relatives.Virol J. 2010 Dec 3;7:359. doi: 10.1186/1743-422X-7-359. Virol J. 2010. PMID: 21129204 Free PMC article. Review.
Cited by
-
In vitro reconstitution of DNA replication initiated by genetic recombination: a T4 bacteriophage model for a type of DNA synthesis important for all cells.Mol Biol Cell. 2019 Jan 1;30(1):146-159. doi: 10.1091/mbc.E18-06-0386. Epub 2018 Nov 7. Mol Biol Cell. 2019. PMID: 30403545 Free PMC article.
-
The Many Roles of Binding Cooperativity in the Control of DNA Replication.Biophys J. 2019 Dec 3;117(11):2043-2046. doi: 10.1016/j.bpj.2019.10.029. Epub 2019 Oct 28. Biophys J. 2019. PMID: 31708164 Free PMC article. No abstract available.
-
Regulation of the bacteriophage T4 Dda helicase by Gp32 single-stranded DNA-binding protein.DNA Repair (Amst). 2015 Jan;25:41-53. doi: 10.1016/j.dnarep.2014.10.002. Epub 2014 Nov 14. DNA Repair (Amst). 2015. PMID: 25481875 Free PMC article.
-
Structural and functional insights into the interaction between the bacteriophage T4 DNA processing proteins gp32 and Dda.Nucleic Acids Res. 2024 Nov 11;52(20):12748-12762. doi: 10.1093/nar/gkae910. Nucleic Acids Res. 2024. PMID: 39417586 Free PMC article.
References
-
- Beernink H. T., Morrical S. W. (1999) RMPs. Recombination/replication mediator proteins. Trends Biochem. Sci. 24, 385–389 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

