Myeloid-derived suppressor cells enhance IgE-mediated mast cell responses
- PMID: 24338630
- PMCID: PMC3958743
- DOI: 10.1189/jlb.0913510
Myeloid-derived suppressor cells enhance IgE-mediated mast cell responses
Abstract
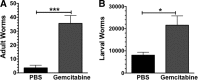
Mast cells and MDSCs are increased by parasitic infection and tumor growth. We previously demonstrated that enhanced MDSC development in ADAM10 transgenic mice yielded resistance to Nb infection and that coculturing MDSCs and mast cells enhanced cytokine production. In the current work, we show that MDSC-mast cell coculture selectively enhances IgE-mediated cytokine secretion among mast cells, without increasing MDSC cytokine production. This effect was independent of cell contact and elicited by Ly6C(+) and Ly6C/G+ MDSC subsets. These interactions were functionally important. MDSC depletion with the FDA-approved drug gemcitabine exacerbated Nb or Trichinella spiralis infection and reduced mast cell-dependent AHR and lung inflammation. Adoptive transfer of MDSC worsened AHR in WT but not mast cell-deficient Wsh/Wsh mice. These data support the hypothesis that MDSCs enhance mast cell inflammatory responses and demonstrate that this interaction can be altered by an existing chemotherapeutic.
Keywords: Nippostrongylus; Trichinella; allergy; asthma; inflammation.
Figures





Similar articles
-
Cutting edge: mast cells critically augment myeloid-derived suppressor cell activity.J Immunol. 2012 Jul 15;189(2):511-5. doi: 10.4049/jimmunol.1200647. Epub 2012 Jun 15. J Immunol. 2012. PMID: 22706087 Free PMC article.
-
Impaired protection against Trichinella spiralis in mice with high levels of IgE.Parasitol Int. 2014 Apr;63(2):332-6. doi: 10.1016/j.parint.2013.12.004. Epub 2013 Dec 15. Parasitol Int. 2014. PMID: 24342553
-
IgE enhances parasite clearance and regulates mast cell responses in mice infected with Trichinella spiralis.J Immunol. 2004 Jan 15;172(2):1139-45. doi: 10.4049/jimmunol.172.2.1139. J Immunol. 2004. PMID: 14707089
-
Role of IgE in the development of allergic airway inflammation and airway hyperresponsiveness--a murine model.Allergy. 1999 Apr;54(4):297-305. doi: 10.1034/j.1398-9995.1999.00085.x. Allergy. 1999. PMID: 10371087 Review.
-
Role of immunoglobulin E and mast cells in murine models of asthma.Braz J Med Biol Res. 2003 Jul;36(7):821-7. doi: 10.1590/s0100-879x2003000700001. Epub 2003 Jun 26. Braz J Med Biol Res. 2003. PMID: 12845367 Review.
Cited by
-
Mast cell/MDSC a liaison immunosuppressive for tumor microenvironment.Oncoimmunology. 2015 Mar 24;4(4):e1001232. doi: 10.1080/2162402X.2014.1001232. eCollection 2015 Apr. Oncoimmunology. 2015. PMID: 26137400 Free PMC article.
-
The Controversial Role of Intestinal Mast Cells in Colon Cancer.Cells. 2023 Jan 31;12(3):459. doi: 10.3390/cells12030459. Cells. 2023. PMID: 36766801 Free PMC article. Review.
-
Therapies for tuberculosis and AIDS: myeloid-derived suppressor cells in focus.J Clin Invest. 2020 Jun 1;130(6):2789-2799. doi: 10.1172/JCI136288. J Clin Invest. 2020. PMID: 32420917 Free PMC article. Review.
-
Here, There, and Everywhere: Myeloid-Derived Suppressor Cells in Immunology.J Immunol. 2023 May 1;210(9):1183-1197. doi: 10.4049/jimmunol.2200914. J Immunol. 2023. PMID: 37068300 Free PMC article. Review.
-
Cross-talk among myeloid-derived suppressor cells, macrophages, and tumor cells impacts the inflammatory milieu of solid tumors.J Leukoc Biol. 2014 Dec;96(6):1109-18. doi: 10.1189/jlb.3A0414-210R. Epub 2014 Aug 28. J Leukoc Biol. 2014. PMID: 25170116 Free PMC article.
References
-
- Kalesnikoff J., Galli S. J. (2010) Anaphylaxis: mechanisms of mast cell activation. Chem. Immunol. Allergy 95, 45–66 - PubMed
-
- Taylor M. D., van der Werf N., Maizels R. M. (2012) T cells in helminth infection: the regulators and the regulated. Trends Immunol. 33, 181–189 - PubMed
-
- Cuervo H., Guerrero N. A., Carbajosa S., Beschin A., De Baetselier P., Girones N., Fresno M. (2011) Myeloid-derived suppressor cells infiltrate the heart in acute Trypanosoma cruzi infection. J. Immunol. 187, 2656–2665 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

