Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems
- PMID: 24338748
- PMCID: PMC4074410
- DOI: 10.1002/jps.23773
Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems
Abstract
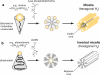
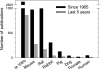
Liposomes are spherical-enclosed membrane vesicles mainly constructed with lipids. Lipid nanoparticles are loaded with therapeutics and may not contain an enclosed bilayer. The majority of those clinically approved have diameters of 50-300 nm. The growing interest in nanomedicine has fueled lipid-drug and lipid-protein studies, which provide a foundation for developing lipid particles that improve drug potency and reduce off-target effects. Integrating advances in lipid membrane research has enabled therapeutic development. At present, about 600 clinical trials involve lipid particle drug delivery systems. Greater understanding of pharmacokinetics, biodistribution, and disposition of lipid-drug particles facilitated particle surface hydration technology (with polyethylene glycol) to reduce rapid clearance and provide sufficient blood circulation time for drug to reach target tissues and cells. Surface hydration enabled the liposome-encapsulated cancer drug doxorubicin (Doxil) to gain clinical approval in 1995. Fifteen lipidic therapeutics are now clinically approved. Although much research involves attaching lipid particles to ligands selective for occult cells and tissues, preparation procedures are often complex and pose scale-up challenges. With emerging knowledge in drug target and lipid-drug distribution in the body, a systems approach that integrates knowledge to design and scale lipid-drug particles may further advance translation of these systems to improve therapeutic safety and efficacy.
Keywords: disposition; drug delivery systems; lipids; liposomes; micelle; nanoparticles; nanotechnology; pegylation; phospholipids.
© 2013 Wiley Periodicals, Inc. and the American Pharmacists Association.
Figures
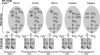

References
-
- Saunders L, Thomas IL. Diffusion studies with lysolecithin. J Chem Soc. 1958:483–485.
-
- Bangham AD, Horne RW. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J Mol Biol. 1964;8:660–668. - PubMed
-
- Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–160. - PubMed
-
- Kinman L, Brodie SJ, Tsai CC, Bui T, Larsen K, Schmidt A, Anderson D, Morton WR, Hu SL, Ho RJ. Lipid–drug association enhanced HIV-1 protease inhibitor indinavir localization in lymphoid tissues and viral load reduction: A proof of concept study in HIV-2287-infected macaques. J Acquir Immune Defic Syndr. 2003;34(4):387–397. - PubMed
-
- James JS. DOXIL approved for KS. AIDS treatment news (no. 236) 1995:6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

