Optimum duration of regimens for Helicobacter pylori eradication
- PMID: 24338763
- PMCID: PMC11841770
- DOI: 10.1002/14651858.CD008337.pub2
Optimum duration of regimens for Helicobacter pylori eradication
Abstract
Background: The optimal duration for Helicobacter pylori (H. pylori) eradication therapy is controversial, with recommendations ranging from 7 to 14 days. Several systematic reviews have attempted to address this issue but have given conflicting results and limited their analysis to proton pump inhibitor (PPI), two antibiotics (PPI triple) therapy. We performed a systematic review and meta-analysis to investigate the optimal duration of multiple H. pylori eradication regimens.
Objectives: The primary objective was to assess the relative effectiveness of different durations (7, 10 or 14 days) of a variety of regimens for eradicating H. pylori. The primary outcome was H. pylori persistence. The secondary outcome was adverse events.
Search methods: The Cochrane Library, MEDLINE, EMBASE, and CINAHL were searched up to December 2011 to identify eligible randomised controlled trials (RCTs). We also searched the proceedings of six conferences from 1995 to 2011, dissertations and theses, and grey literature. There were no language restrictions applied to any search.
Selection criteria: Only parallel group RCTs assessing the efficacy of one to two weeks duration of first line H. pylori eradication regimens in adults were eligible. Within each regimen, the same combinations of drugs at the same dose were compared over different durations. Studies with at least two arms comparing 7, 10, or 14 days were eligible. Enrolled participants needed to be diagnosed with at least one positive test for H. pylori on the basis of a rapid urease test (RUT), histology, culture, urea breath test (UBT), or a stool antigen test (HpSA) before treatment. Eligible trials needed to confirm eradication of H. pylori as their primary outcome at least 28 days after completion of eradication treatment. Trials using only serology or a polymerase chain reaction (PCR) to determine H. pylori infection or eradication were excluded.
Data collection and analysis: Study eligibility and data extraction were performed by two independent review authors. Data analyses were performed within each type of intervention, for both primary and secondary outcomes. The relative risk (RR) and number needed to treat (NNT)/number needed to harm (NNTH) according to duration of therapy were calculated using the outcomes of H. pylori persistence and adverse events. A random-effects model was used. Subgroup analyses and sensitivity analyses were planned a priori.
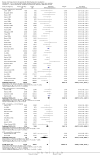
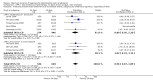
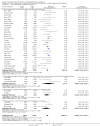
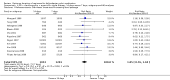
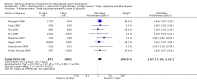
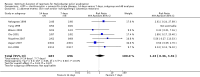
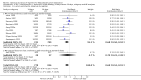
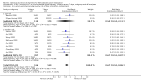
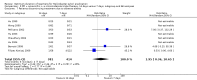
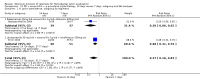
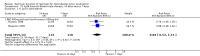
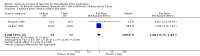
Main results: In total, 75 studies met the inclusion criteria. Eight types of regimens were reported with at least two comparative eligible durations. They included: PPI + two antibiotics triple therapy (n = 59), PPI bismuth-based quadruple therapy (n = 6), PPI + three antibiotics quadruple therapy (n = 1), PPI dual therapy (n = 2), histamine H2-receptor antagonist (H₂RA) bismuth quadruple therapy (n = 3), H₂RA bismuth-based triple therapy (n = 2), H₂RA + two antibiotics triple therapy (n = 3), and bismuth + two antibiotics triple therapy (n = 2). Some studies provided data for more than one regimen or more than two durations.For the PPI triple therapy, 59 studies with five regimens were reported: PPI + clarithromycin + amoxicillin (PCA); PPI + clarithromycin + a nitroimidazole (PCN); PPI + amoxicillin + nitroimidazole (PAN); PPI + amoxicillin + a quinolone (PAQ); and PPI + amoxicillin + a nitrofuran (PANi). Regardless of type and dose of antibiotics, increased duration of PPI triple therapy from 7 to 14 days significantly increased the H. pylori eradication rate (45 studies, 72.9% versus 81.9%), the RR for H. pylori persistence was 0.66 (95% CI 0.60 to 0.74), NNT was 11 (95% CI 9 to 14). Significant effects were seen in the subgroup of PCA (34 studies, RR 0.65, 95% CI 0.57 to 0.75; NNT 12, 95% CI 9 to 16); PAN (10 studies, RR 0.67, 95% CI 0.52 to 0.86; NNT = 11, 95% CI 8 to 25); and in PAQ (2 studies, RR 0.37, 95% CI 0.16 to 0.83; NNT 3, 95% CI 2 to 10); but not in PCN triple therapy (4 studies, RR 0.87, 95% CI 0.71 to 1.07). Significantly increased eradication rates were also seen for PPI triple therapy with 10 versus 7 days (24 studies, 79.9% versus 75.7%; RR 0.80, 95% CI 0.72 to 0.89; NNT 21, 95% CI 15 to 38) and 14 versus 10 days (12 studies, 84.4% versus 78.5%; RR 0.72, 95% CI 0.58 to 0.90; NNT 17, 95% CI 11 to 46); especially in the subgroup of PAC for 10 versus 7 days (17 studies, RR 0.80, 95% CI 0.70 to 0.91) and for 14 versus 10 days (10 studies, RR 0.69, 95% CI 0.52 to 0.91). A trend towards increased H. pylori eradication rates was seen with increased duration of PCN for 10 versus 7 days, and of PAN for 10 versus 7 days and 14 versus 10 days, though this was not statistical significant. The proportion of patients with adverse events, defined by authors, was marginally significantly increased only between 7 days and 14 days (15.5% versus 19.4%; RR 1.21, 95% CI 1.06 to 1.37; NNTH 31, 95% CI 18 to 104) but not for other duration comparisons. The proportion of patients discontinuing treatment due to adverse events was not significantly different between treatment durations.Only limited data were reported for different durations of regimens other than PPI triple therapy. No significant difference of the eradication rate was seen for all regimens according to different durations except for H₂RA bismuth quadruple therapy, where a significantly higher eradication rate was seen for 14 days versus 7 days, however only one study reported outcome data.
Authors' conclusions: Increasing the duration of PPI-based triple therapy increases H. pylori eradication rates. For PCA, prolonging treatment duration from 7 to 10 or from 10 to 14 days is associated with a significantly higher eradication rate. The optimal duration of therapy for PCA and PAN is at least 14 days. More data are needed to confirm if there is any benefit of increasing the duration of therapy for PCN therapy. Information is limited for regimens other than PPI triple therapy; more studies are needed to draw meaningful conclusions for optimal duration of other H. pylori eradication regimens.
Conflict of interest statement
Research ethics
This study does not involve any experiments performed on human or animal bodies or tissues. Only published literature were collected and analysed. No direct harm will be caused by this study therefore no research ethnic board approval is required by the McMaster University Research Ethics Board. However, the analysis results may have impact on future decision making in the clinical practice which is believed no harm would be caused.
Funding source
No funding is received in respect of this study.
Conflict of interest
Yuhong Yuan, Alex Ford, Khurram Khan and Xavier Calvet declare no conflict of interest for this study. Dr Gisbert has served as a speaker, a consultant and advisory member for, or has received research funding from Almirall, AstraZeneca, Janssen‐Cilag, and Nycomed. In the last two years, Dr Carlo Fallone has received funding for consulting from Pendopharm Canada, and Takeda. Paul Moayyedi has accepted speaker's fees from AstraZeneca and Shire over the last two years and his Chair is partly funded by an unrestricted donation from AstraZeneca to McMaster University.
Figures
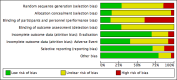





















































































































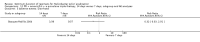
















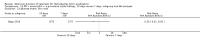
























































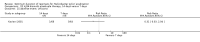













































Comment in
-
[Helicobacter pylori eradication: best treated for 14 days].Dtsch Med Wochenschr. 2014 Apr;139(15):764. doi: 10.1055/s-0033-1360607. Epub 2014 Apr 1. Dtsch Med Wochenschr. 2014. PMID: 24691687 German. No abstract available.
-
Optimal Duration of Treatment Regimens for H. pylori Eradication.Am Fam Physician. 2014 Dec 1;90(11):766-7. Am Fam Physician. 2014. PMID: 25611710 No abstract available.
References
References to studies included in this review
Antelo 2001 {published data only}
-
- Antelo P, Almuzara M, Avagnina A, Topor J, Barberis C, Barcia T, et al. Diagnosis and treatment of Helicobacter pylori infection. Relationship with gastrointestinal ulcer and antimicrobial resistance [Diagnostico y tratamiento de la infeccion por Helicobacter Pylori su relacion con la ulcera gastrointestinal y la resistencia a los antimicrobianos]. Medicina (B Aires) 2001;61(5 part 1):545‐51. - PubMed
Aydin 2007 {published data only}
-
- Aydin A, Onder G, Akarca U, Tekin F, Tuncyurek M, Ilter T. Comparison of 1‐ and 2‐week pantoprazole‐based triple therapies in clarithromycin‐sensitive and resistant cases. European Journal of Internal Medicine 2007;18(6):496‐500. - PubMed
Bago 2010 {published data only}
-
- Bago J, Majstorović K, Belosić‐Halle Z, Kućisec N, Bakula V, Tomić M, et al. Antimicrobial resistance of H. pylori to the outcome of 10‐days vs. 7‐days Moxifloxacin based therapy for the eradication: a randomized controlled trial. Annals of Clinical Microbiology and Antimicrobials 2010;9(13):1‐6. - PMC - PubMed
Basu 2011 {published data only}
Berrutti 2008 {published data only}
-
- Berrutti M, Pellicano R, Astegiano M, Smedile A, Saracco G, Morgando A, et al. Helicobacter pylori eradication: metronidazole or tinidazole? Data from Turin, Italy. Minerva Gastroenterologica e Dietologica 2008;54(4):355‐8. - PubMed
Bhasin 2000 {published data only}
-
- Bhasin DK, Sharma BC, Ray P, Pathak CM, Singh K. Comparison of seven and fourteen days of lansoprazole, clarithromycin, and amoxicillin therapy for eradication ofHelicobacter pylori: a report from India. Helicobacter 2000;5(2):84‐7. - PubMed
Bosques‐Padilla 2004 {published data only}
-
- Bosques‐Padilla FJ, Garza‐González E, Calderón‐Lozano IE, Reed‐SanRoman G, Ariño Suárez M, Valdovinos‐Díaz MA, et al. Open, randomized multicenter comparative trial of rabeprazole, ofloxacin and amoxicillin therapy for Helicobacter pylori eradication: 7 vs. 14 day treatment. Helicobacter 2004;9(5):417‐21. - PubMed
Calvet 2005a {published data only}
-
- Calvet X, Ducons J, Bujanda L, Bory F, Montserrat A, Gisbert JP, et al. Seven versus ten days of rabeprazole triple therapy for Helicobacter pylori eradication: a multicenter randomized trial. American Journal of Gastroenterology 2005;100(8):1696‐701. - PubMed
Chaudhary 2004 {published data only}
-
- Chaudhary A, Ahuja V, Bal CS, Das B, Pandey RM, Sharma MP. Rank order of success favors longer duration of imidazole‐based therapy for Helicobacter pylori in duodenal ulcer disease: a randomized pilot study. Helicobacter 2004;9(2):124‐9. - PubMed
Chew 1994 {published data only}
-
- Chew K, Eidus L. Clarithromycin and high dose omeprazole for H. pylori eradication: a comparison on one and two week therapy. Gastroenterology 1994;106 (4, Pt2):A 62.
Ching 1998 {published data only}
-
- Ching CK, Chan YK, Ng WC. The combination of omeprazole, amoxycillin, and clarithromycin eradicates Helicobacter pylori in 95% of patients ‐‐7 days of therapy is as good as 10 days. Hong Kong Medical Journal 1998;4(1):7‐10. - PubMed
Cho 2001 {published data only}
-
- Cho YJ, Chun HJ, Kim ST, Koh DW, Park JH, Park DK, et al. Analysis of eradication rate of Helicobacter pylori according to treatment duration by using 13C‐Urea breath test comparison of OAC 7, 10 or 14 days regimen. [Korean]. Korean Journal of Gastrointestinal Endoscopy 2001;23(4):207‐12.
Cui 2002 {published data only}
-
- Cui M. Hu F. Jiang H. Comparison of pantoprazole and omeprazole‐based triple therapy regimens in the treatment of Helicobacter pylori infection. [Chinese] [泮托拉唑三联与奥美拉唑三联疗法根除幽门螺杆菌的对比研究]. Zheng X Zhonghua Yi Xue Za Zhi (Natlonal Medical Journal of China) 2002;82(18):1245‐8. - PubMed
Daghaghzadeh 2007 {published data only}
-
- Daghaghzadeh H, Emami MH, Karimi S, Raeisi M. One‐week versus two‐week furazolidone‐based quadruple therapy as the first‐line treatment for Helicobacter pylori infection in Iran. Journal of Gastroenterology and Hepatology 2007;22(9):1399‐403. - PubMed
Dal Bo' 1998 {published data only}
-
- Dal Bo' N, Mario F, Battaglia G, Buda A, Leandro G, Vianello F, et al. Low dose of clarithromycin in triple therapy for the eradication of Helicobacter pylori: one or two weeks?. Journal of Gastroenterology and Hepatology 1998;13(3):288‐93. - PubMed
Dammann 2000 {published data only}
-
- Dammann HG, Folsch UR, Hahn EG, Kleist DH, Klör HU, Kirchner T, et al. Eradication of H. pylori with pantoprazole, clarithromycin, and metronidazole in duodenal ulcer patients: a head‐to‐head comparison between two regimens of different duration. Helicobacter 2000;5(1):41‐51. - PubMed
de Boer 1994 {published data only}
-
- Boer WA, Driessen WM, Potters VP, Tytgat GN. Randomized study comparing 1 with 2 weeks of quadruple therapy for eradicating Helicobacter pylori. American Journal of Gastroenterology 1994;89(11):1993‐7. - PubMed
De Francesco 2004 {published data only}
-
- Francesco V, Zullo A, Hassan C, Della Valle N, Pietrini L, Minenna MF, et al. The prolongation of triple therapy for Helicobacter pylori does not allow reaching therapeutic outcome of sequential scheme: a prospective, randomised study. Digestive and Liver Disease 2004;36(5):322‐6. - PubMed
Di Caro 2002a {published data only}
-
- Caro S, Zocco MA, Cremonini F, Candelli M, Nista EC, Bartolozzi F, et al. Levofloxacin based regimens for the eradication of Helicobacter pylori. European Journal of Gastroenterology and Hepatology 2002;14(12):1309‐12. - PubMed
Di Mario 2003a {published data only}
-
- Mario F, Aragona G, Dal Bo N, Cavestro GM, Cavallaro L, Iori V, et al. Use of bovine lactoferrin for Helicobacter pylori eradication. Digestive and Liver Disease 2003;35(10):706‐10. - PubMed
Dore 2011 {published data only}
-
- Dore MP, Farina V, Cuccu M, Mameli L, Massarelli G, Graham DY. Twice‐a‐day bismuth‐containing quadruple therapy for Helicobacter pylori eradication: a randomized trial of 10 and 14 days. Helicobacter 2011;16(4):295‐300. - PubMed
Emami 2006 {published data only}
Ercin 2010 {published data only}
-
- Erçin CN, Uygun A, Toros AB, Kantarcioğlu M, Kilciler G, Polat Z, Bağci S. Comparison of 7‐ and 14‐day first‐line therapies including levofloxacin in patients with Helicobacter pylori positive non‐ulcer dyspepsia. Turkish Journal of Gastroenterology 2010;21(1):12‐6. - PubMed
Fennerty 1998 {published data only}
-
- Fennerty MB, Kovacs TO, Krause R, Haber M, Weissfeld A, Siepman N, et al. A comparison of 10 and 14 days of lansoprazole triple therapy for eradication ofHelicobacter pylori. Archives of Internal Medicine 1998;158(15):1651‐6. - PubMed
Filipec Kanizaj 2009 {published data only}
-
- Filipec Kanizaj T, Katicic M, Skurla B, Ticak M, Plecko V, Kalenic S. Helicobacter pylori eradication therapy success regarding different treatment period based on clarithromycin or metronidazole triple‐therapy regimens. Helicobacter 2009;14(1):29‐35. - PubMed
Garza González 2007 {published data only}
-
- Garza González E, Giasi González E, Martínez Vázquez MA, Pérez Pérez GI, González GM, Maldonado Garza HJ, Bosques Padilla FJ. Helicobacter pylori eradication and its relation to antibiotic resistance and CYP2C19 status. Revista Espanola de Enfermedades Digestivas 2007;99(2):71‐5. - PubMed
Gisbert 2005a {published data only}
-
- Gisbert JP, Dominguez‐Munoz A, Dominguez‐Martin A, Gisbert JL, Marcos S. Esomeprazole‐based therapy in Helicobacter pylori eradication: any effect by increasing the dose of esomeprazole or prolonging the treatment?. American Journal of Gastroenterology 2005;100(9):1935‐40. - PubMed
Grade 1996 {published data only}
-
- Grade AJ, Blubaugh FC, Ramirez FC. Clarithromycin, amoxicillin and omeprazole, for eradication of H. pylori: a prospective, randomized, comparative study of a seven day versus ten day course. Gastroenterology 1996;110 Suppl 1:A121.
Gumurdulu 2004 {published data only}
Hsu 2005 {published data only}
-
- Hsu CC, Chen JJ, Hu TH, Lu SN, Changchien CS. One‐week versus two‐week H2‐receptor antagonist in combination with amoxicillin and tinidazole for eradication of Helicobacter pylori infection. Hepato‐Gastroenterology 2005;52(65):1617‐21. - PubMed
Hu 1999 {published data only}
-
- Hu F, Wang Z, Fan G. Comparison of 1 week and 2 weeks efficacy of lansoprazole triple therapy in treatment of H. pylori infection [兰索拉唑三联疗法根除幽门螺杆菌1周与2周疗效对比研究]. Chinese New Drugs Journal 1999;8(4):246‐8.
Hu 2003 {published data only}
-
- Hu FL, Jia JC, Li YL, Yang GB. Comparison of H2‐receptor antagonist‐ and proton‐pump inhibitor‐based triple regimens for the eradication of Helicobacter pylori in Chinese patients with gastritis or peptic ulcer. The Journal of International Medical Research 2003;31(6):469‐74. - PubMed
Hu 2004 {published data only}
-
- Hu FL for Multiple Center Study Group In Beijing Area, China. Effects of different triple therapies on duodenal ulcer‐associated Helicobacter pylori infection and a one‐year follow‐up study [不同组合的三联疗法对幽门螺杆菌阳性十二指肠溃疡的疗效及一年随访研究]. Zhonghua Yi Xue Za Zhi (National Medical Journal of China) 2004;84(14):1161‐5. - PubMed
Hurenkamp 2000 {published data only}
-
- Hurenkamp GJ, Ende A, Grundmeijer HG, Tytgat GN, Hulst RW. Equally high efficacy of 4, 7 and 10‐day triple therapies to eradicate Helicobacter pylori infection in patients with ulcer disease. Alimentary Pharmacology and Therapeutics 2000;14(8):1065‐70. - PubMed
Kang 2000a {published data only}
-
- Kang CD, Chun HJ, Park DK, Hur BW, Lee JW, Jee YT, et al. Significance of pretreatment of delta 13CO2 as predictor of success for H. pylori eradication. Gut 2000;47 Suppl 3:A120. Abstract P365.
Karatapanis 2011 {published data only}
-
- Karatapanis S, Georgopoulos SD, Papastergiou V, Skorda L, Papantoniou N, Lisgos P, et al. 7, 10 and 14‐days rabeprazole‐based standard triple therapies for H. pylori eradication: are they still effective? A randomized trial. Acta Gastroenterologica Belgica 2011;74(3):407‐12. - PubMed
Katicic 1997 {published data only}
-
- Katicic M, Presecki V, Marusic M, Prskalo M, Ticak M, Sabaric B, et al. Eradication of H. pylori infection with two triple‐therapy regimens of 7, 10 and 14 days. Gut 1997;41 Suppl 1:A100. Abstract#09/367.
Kaviani 2001 {published data only}
-
- Kaviani MJ, Malekzadeh R, Vahedi H, Sotoudeh M, Kamalian N, Amini M, et al. Various durations of a standard regimen (amoxycillin, metronidazole, colloidal bismuth sub‐citrate for 2 weeks or with additional ranitidine for 1 or 2 weeks) on eradication of Helicobacter pylori in Iranian peptic ulcer patients. A randomized controlled trial. European Journal of Gastroenterology and Hepatology 2001;13(8):915‐9. - PubMed
Keum 2005 {published data only}
-
- Keum B, Lee SW, Kim SY, Kim MJ, Choung RS, Yim HJ, et al. Comparison of Helicobacter pylori eradication rate according to different PPI‐based triple therapy: omeprazole, rabeprazole, Esomeprazole and lansoprazol (in Korean). Korean Journal of Gastroenterology 2005;46(6):433‐9. - PubMed
Kim 2007 {published data only}
-
- Kim BG, Lee DH, Ye BD, Lee KH, Kim BW, Kim SG, et al. Comparison of 7‐day and 14‐day proton pump inhibitor‐containing triple therapy for Helicobacter pylori eradication: neither treatment duration provides acceptable eradication rate in Korea. Helicobacter 2007;12(1):31‐5. - PubMed
Kim 2008 {published data only}
-
- Kim N, Park SH, Seo GS, Lee SW, Kim JW, Lee KJ, et al. Lafutidine versus lansoprazole in combination with clarithromycin and amoxicillin for one versus two weeks for Helicobacter pylori eradication in Korea. Helicobacter 2008;13(6):542‐9. - PubMed
Kiyota 1999 {published data only}
-
- Kiyota K, Habu Y, Sugano Y, Inokuchi H, Mizuno S, Kimoto K, et al. Comparison of 1‐week and 2‐week triple therapy with omeprazole, amoxicillin, and clarithromycin in peptic ulcer patients with Helicobacter pylori infection: results of a randomized controlled trial. Journal of Gastroenterology 1999;34 Suppl XI:76‐9. - PubMed
Knigge 1999 {published data only}
-
- Knigge K, Kelly C, Peterson WL, Fennerty MB. Eradication of Helicobacter pylori infection after ranitidine bismuth citrate, metronidazole and tetracycline for 7 or 10 days. Alimentary Pharmacology and Therapeutics 1999;13(3):323‐6. - PubMed
Laine 1996 {published data only}
-
- Laine L, Estrada R, Trujillo M, Fukanaga K, Neil G. Randomized comparison of differing periods of twice‐a‐day triple therapy for the eradication of Helicobacter pylori. Alimentary Pharmacology and Therapeutics 1996;10(6):1029‐33. - PubMed
Lee 2004 {published data only}
-
- Lee D, Lee S, Chun H, Jung H, Kim J, Korean Helicobacter pylori study group. Two weeks triple regimens with PPI, clarithromycin and amoxicillin is more effective in H. pylori eradication than one week triple regimens in patients with peptic ulcer disease in South Korea. (Multicenter trial by Korean Helicobacter pylori Study Group). Helicobacter 2004;9 (5):570. Abstract #11.08.
Louw 1998a {published data only}
-
- Louw JA, Rensburg CJ, Moola S, Kotze D, Marks IN. Helicobacter pylori eradication and ulcer healing with daily lansoprazole, plus 1 or 2 weeks co‐therapy with amoxycillin and clarithromycin. Alimentary Pharmacology and Therapeutics 1998;12(9):881‐5. - PubMed
Louw 1998b {published data only}
-
- Louw JA, Rensburg CJ, Hanslo D, Grundlings HD, Girdwood AH, Marks IN. Two‐week course of pantoprazole combined with 1 week of amoxycillin and clarithromycin is effective in Helicobacter pylori eradication and duodenal ulcer healing. Alimentary Pharmacology and Therapeutics 1998;12(6):545‐50. - PubMed
Lv 2011 {published data only}
-
- Lv NH, Xie Y, Guo XB, Fan HZ, Tang JH, Zeng XP, et al. Eradication therapy for Helicobacter pylori infection in patients with duodenal ulcers based on furazolidone triple and quadruple therapy: A multicenter randomized controlled trial. Helicobacter 2011;16 Suppl 1:87 Abstract #WS5.3.
Maconi 2001 {published data only}
-
- Maconi G, Parente F, Russo A, Vago L, Imbesi V, Bianchi Porro G. Do some patients with Helicobacter pylori infection benefit from an extension to 2 weeks of a proton pump inhibitor‐based triple eradication therapy?. American Journal of Gastroenterology 2001;96(2):359‐66. - PubMed
-
- Maconi G, Russo A, Imbesi V, Cucino C, Bianchi Porro G. Prolonging proton pump inhibitor‐based anti‐Helicobacter pylori treatment from one to two weeks in duodenal ulcer: is it worthwhile?. Digestive and Liver Disease 2000;32(4):275‐80. - PubMed
Mantzaris 1999 {published data only}
-
- Mantzaris GJ, Tzathas H, Petrak K, Spiliades C, Archavlis E, Amberiadis P, et al. A prospective study comparing various anti‐H. pylori treatment regimens for patients with peptic ulcer disease (PUD). Gut 1999;45 Suppl 5:A192 Abstract #P0578.
Mesquita 2005 {published data only}
-
- Mesquita MA, Lorena SL, Almeida JR, Montes CG, Guerrazzi F, Campos LT, et al. One‐week dual therapy with ranitidine bismuth citrate and clarithromycin for the treatment of Helicobacter pylori infection in Brazilian patients with peptic ulcer. World Journal of Gastroenterology 2005;11(23):3566‐9. - PMC - PubMed
Moayyedi 1996 {published data only}
-
- Moayyedi P, Langworthy H, Shanahan K, Tompkins DS, Dixon MF, Chalmers DM, et al. Comparison of one or two weeks of lansoprazole, amoxicillin, and clarithromycin in the treatment of Helicobacter pylori. Helicobacter 1996;1(2):71‐4. - PubMed
Mones 1997 {published data only}
-
- Mones J, Sainz S, Sola‐Vera J, Richart E, Sancho FJ, Balanzo J. Helicobacter pylori eradication with omeprazole, amoxicillin and clarithromycin: One week vs two weeks and reinfection rate at one year. Gastroenterology 1997;112(4):A224.
Muszynki 1992 {published data only}
-
- Muszynski J, Czyzyk A, Biernacka D, Pachecka J, Stepka M, Zalewski L. Combined treatment of Helicobacter pylori infection‐‐7‐day or 14‐day treatment? [Skojarzone leczenie zakazenia Helicobacter pylori‐terapia 7 czy 14‐dniowa?]. Polski Tygodnik Lekarski 1992;47(29‐30):642‐5. - PubMed
Nakagawa 1999 {published data only}
-
- Nakagawa M, Ooishi M, Yoda Y, Kawamura N, Ooizumi H, Saitou M, Nakagawa S. A comparative study; one week treatment versus two weeks treatment with lansoprazole, amoxycillin and clarithromycin for the eradication of Helicobacter pylori in patient with gastric and duodenal ulcer [Japanese]. Nippon Rinsho 1999;57(1):144‐7. - PubMed
Palmas 2002 {published data only}
-
- Palmas F, Pellicano R, Massimetti E, Berrutti M, Fagoonee S, Rizzetto M. Eradication of Helicobacter pylori infection with proton pump inhibitor‐based triple therapy. A randomised study. Panminerva Medica 2002;44(2):145‐7. - PubMed
Paoluzi 1998 {published data only}
-
- Paoluzi P, Rossi P, Consolazio A, Paoluzi OA, Pietroiusti A, Maggio F, et al. A single‐blind, monocentric study of four treatments for H. pylori eradication: an interim report. Gastroenterology 1998;114(4 Suppl 2):A252‐3. Abstract G1039.
Paoluzi 2006 {published data only}
-
- Paoluzi P, Iacopini F, Crispino P, Nardi F, Bella A, Rivera M, et al. 2‐week triple therapy for Helicobacter pylori infection is better than 1‐week in clinical practice: a large prospective single‐center randomized study. Helicobacter 2006;11(6):562‐8. - PubMed
Park 2009a {published and unpublished data}
-
- Park SH, Chun HJ, Choi HS, Lee KG, Kim JH, Hyun JJ, et al. The 10‐day sequential therapy for Helicobacter pylori eradication in Korea: Less effective than expected. Gut 2009;58 Suppl II:A256. Abstract#P0726.
Pellicano 2002 {published data only}
-
- Pellicano R, Palmas F, Ponzetto A, Astegiano M, Smedile A, Morgando A, et al. Decreasing eradication rate of Helicobacter pylori infection with metronidazole‐based triple therapy. A randomised study. Minerva Gastroenterologica e Dietologica 2002;48(3):265‐70. - PubMed
Pozzato 1998 {published data only}
-
- Pozzato P, Zagari M, Cardelli A, Catalano FA, Giglio A, Lami F, et al. Ranitidine bismuth citrate plus clarithromycin 7‐day regimen is effective in eradicating Helicobacter pylori in patients with duodenal ulcer. Alimentary Pharmacology and Therapeutics 1998;12(5):447‐51. - PubMed
Riquelme 2007 {published data only}
Robles‐Jara 2008 {published data only}
Sacco 2010 {published data only}
-
- Sacco F, Spezzaferro M, Amitrano M, Grossi L, Manzoli L, Marzio L. Efficacy of four different moxifloxacin‐based triple therapies for first‐line H. pylori treatment. Digestive and Liver Disease 2010;42(2):110‐4. - PubMed
Savarino 1999 {published data only}
-
- Savarino V, Zentilin P, Bisso G, Pivari M, Bilardi C, Biagini R, et al. Optimal duration of therapy combining ranitidine bismuth citrate with clarithromycin and metronidazole in the eradication of Helicobacter pylori infection. Alimentary Pharmacology and Therapeutics 1999;13(1):43‐7. - PubMed
Scaccianoce 2006 {published data only}
Scaccianoce 2008 {published data only}
-
- Scaccianoce G, Zullo A, Hassan C, Gentili F, Cristofari F, Cardinale V, et al. Triple therapies plus different probiotics for Helicobacter pylori eradication. European Review for Medical and Pharmacological Sciences 2008;12(4):251‐6. - PubMed
Simsek 1999 {published data only}
-
- Simsek I, Soyturk M, Akpinar H, Ellidokuz E, Topalak O, Okan E, et al. The comparison of the efficiency of the efficiency of one and two weeks lansoprazole, amoxycillin, clarithromycin (LAC) treatment protocols in the eradication of Helicobacter Pylori by using urea breath test. Gut 1999;45 Suppl 5:A274. Abstract #P1037.
Sun 2010 {published data only}
-
- Sun Q, Liang X, Zheng Q, Liu W, Xiao S, Gu W, Lu H. High efficacy of 14‐day triple therapy‐based, bismuth‐containing quadruple therapy for initial Helicobacter pylori eradication. Helicobacter 2010;15(3):233‐8. - PubMed
Suriani 2008 {published data only}
-
- Suriani R, Serra A, Vernetto A, Mazzucco D, Grosso S, Morgando A, et al. Probiotic supplementation improves tolerance to H. pylori eradication therapy in dyspeptic patients: preliminary data of a multicenter, controlled, double‐blind randomized pilot study. Helicobacter 2008;13 (5):470.
Vakil 2004 {published data only}
-
- Vakil N, Lanza F, Schwartz H, Barth J. Seven‐day therapy for Helicobacter pylori in the United States. Alimentary Pharmacology and Therapeutics 2004;20(1):99‐107. - PubMed
Wong 2000 {published data only}
-
- Wong BC, Xiao SD, Hu FL, Qian SC, Huang NX, Li YY, et al. Comparison of lansoprazole‐based triple and dual therapy for treatment of Helicobacter pylori‐related duodenal ulcer: an Asian multicentre double‐blind randomized placebo controlled study. Alimentary Pharmacology and Therapeutics 2000;14(2):217‐24. - PubMed
Yang 1999 {published data only}
-
- Yang JC, Yang KC, Hsu CT, Wang CS, Kuo CF, Wang TH. A multicenter study on eradication of Helicobacter pylori infection in patients with duodenal ulcer by lansoprazole‐antibiotics combined therapy. Journal of Microbiology, Immunology, and Infection 1999;32(1):1‐8. - PubMed
Zagari 2007 {published data only}
Zheng 2009 {published data only}
-
- Zheng Q, Dai J, Li X, Lu H, Xiao S. Comparison of the efficacy of pantoprazole based triple therapy versus quadruple therapy in the treatment of Helicobacter pylori infection: A single‐center, randomized, open and parallel‐controlled study [以泮托拉唑为基础的三联和四联疗法根除幽门螺杆菌疗效比较一项单中心、随机、开放、平行对照研究]. Chinese Journal of Gastroenterology 2009;14(1):8–11.
References to studies excluded from this review
Aragona 2002 {published data only}
-
- Aragona G, Bo N, Anna I, Cavestro GM, Moussa AM, Iori V, et al. Lactoferrin in a 7 days triple therapy forHelicobacter Pylori eradication: results of an open randomized trial. Gut 2002;51 Suppl 3:A 109. Abstract MON‐G‐408.
Aragona 2003a {published data only}
-
- Aragona G, Mario F, Cavallaro LG, Cavestro GM, Iori V, Comparato G, et al. Lactoferrin in a 1‐Week Triple Therapy for Eradication of H. pylori. Helicobacter 2003;8:463. Abstract 16.06.
Aragona 2003b {published data only}
-
- Aragona G, Dal Bo' N, Comparato G, Leandro G, Carloni C, Liatopoulou S, et al. Lactoferrin in a one week triple therapy for eradication of H. pylori. Gastroenterology 2003;124(4 Suppl 1):Abstract M1505.
Armuzzi 2001 {published data only}
-
- Armuzzi A, Cremonini F, Ojetti V, Bartolozzi F, Canducci F, Candelli M, et al. Effect of Lactobacillus GG supplementation on antibiotic‐associated gastrointestinal side effects during Helicobacter pylori eradication therapy: a pilot study. Digestion 2001;63(1):1‐7. - PubMed
Basu 2009a {published data only}
-
- Basu PP, Rayapudi K, Pacana T, Shah NJ. A randomized open‐label clinical trial with levofloxacin, omeprazole, Alinia (nitazoxanide) and doxycycline (LOAD) versus lansoprazole, amoxicillin and clarithromycin (LAC) in the treatment naive Helicobacter pylori population. American Journal of Gastroenterology 2009;104 Suppl 3:S404. Abstract 1100.
Basu 2009b {published data only}
-
- Basu PP, Rayapudi K, Pacana T, Ramamurthy S. A randomized open‐label clinical trial with Levofloxacin, Omeprazole, Alinia (nitazoxanide), and Doxycycline (LOAD) versus Lansoprazole, Amoxicillin and Clarithromycin (LAC) in the treatment naive Helicobacter pylori population. Gastroenterology 2009;136(5 Suppl 1):A24. Abstract 131.
Bazzoli 1999 {published data only}
Bazzoli 2000a {published data only}
-
- Bazzoli F, Bianchi Porro G, Fiocca R, Gasbarrinini G, Roda E, Zagari RM. Eradication of Helicobacter pylori and ulcer healing in patients with duodenal ulcer by one‐week or two week omeprazole triple therapy. Gut 2000;46 Suppl 2:Abstract TH49.
Bazzoli 2000b {published data only}
-
- Bazzoli F, Bianchi Porro G, Roda E, Gasbarrini GB, Zagari RM, on behalf of the participants at the HYPER study group. Efficacy of omeprazole plus amoxicillin or amoxicillin plus clarithromycin for 1 week or 2 weeks for helicobacter pylori eradication in patients with duodenal ulcer. Gastroenterology 2000;118 Suppl 2:Abstract 2651.
Bell 1995 {published data only}
-
- Bell GD, Powell KU, Burridge SM, Bowden AF, Atoyebi W, Bolton GH, et al. Rapid eradication of Helicobacter pylori infection. Alimentary Pharmacology and Therapeutics 1995;9(1):41‐6. - PubMed
Boudjella 2009 {published data only}
-
- Boudjella M, Matougui N, Tebaibia A, Oumnia N, Lahcene M, Mouffok F, et al. Factors influencing the efficacy of first‐line treatment of H. pylori injection in Algerian patients. Helicobacter 2009;14(4):350.
Brecken 1999 {published data only}
-
- Brecken RK, Valle PC, Gangsoey Kristiansen M, Nordgaaard K, Wessel‐Berg AM, Bogsrud TV. Tolerance, duration and efficacy of 5 and 7 days low‐dose proton pump inhibitor triple Helicobacter pylori therapy compared to 10 days bismuth triple therapy. Scandinavian Journal of Gastroenterology. Supplement 1999;229:8.
Calderon 2002 {published data only}
-
- Calderon IE, Reed G, Orozco A, Blancas JM, Arino M, Tamayo JL, et al. Open‐label, multicenter, comparative study of therapy with triple drug trial for eradication of Helicobacter pylori with sodium rabeprazole, ofloxacin and amoxicillin. Comparative trial of 7 vs. 14 days treatment. European Helicobacter Study Group. XVth International Workshop 2002; abstract book. 2002:A98. Abstract 15.33.
Calvet 2000 {published data only}
-
- Calvet X, García N, López T, Gisbert JP, Gené E, Roque M. A meta‐analysis of short versus long therapy with a proton pump inhibitor, clarithromycin and either metronidazole or amoxycillin for treating Helicobacter pylori infection. Alimentary Pharmacology and Therapeutics 2000;14(5):603‐9. - PubMed
Calvet 2001 {published data only}
-
- Calvet X, Gené E, López T, Gisbert JP. What is the optimal length of proton pump inhibitor‐based triple therapies for H. pylori? A cost‐effectiveness analysis. Alimentary Pharmacology and Therapeutics 2001;15(7):1067‐76. - PubMed
Calvet 2004 {published data only}
-
- Calvet X, Helicobacter pylori working group of the Asociacion Espanola de Gastroenterologia (AEG). Randomised controlled trial comparing 7 vs. 10 days of triple therapy using rabeprazole, clarithromycin and amoxicillin for Helicobacter pylori (Hp) eradication. Preliminary results. Helicobacter 2004;9:Abstract 11.13.
Calvet 2005b {published data only}
-
- Calvet X, Ducons J, Bujanda L, Bory F, Montserrat A, Gisbert JP. Seven vs. ten‐day rabeprazole triple therapy for Helicobacter Pylori eradication: a multicenter, randomized trial. Gastroenterology 2005;128(4 Suppl 2):A425. Abstract T945. - PubMed
Cardelli 1997 {published data only}
-
- Cardelli A, Cordiano C, Giglio A, Lami F, Pilotto A, Pozzato P, et al. A new dual 7‐day therapy is effective in eradicating Helicobacter pylori in duodenal ulcer patients. Gut 1997;41 Suppl 3:Abstract P750.
Castro 2001 {published data only}
-
- Castro E, Galindo E, for PANTOPYLORI. Efficacy on eradication of H. pylori with three different schedules. Gastroenterology 2001;120(5 Suppl 1):A‐581. Abstract #2952.
Cerqueira 2011 {published data only}
-
- Cerqueira RM, Manso MC, Correia MR, Fernandes CD, Vilar H, Nora M, Martins P. Helicobacter pylori eradication therapy in obese patients undergoing gastric bypass surgery‐‐fourteen days superior to seven days?. Obesity Surgery 2011;21(9):1377‐81. - PubMed
Chen 1995 {published data only}
-
- Chen TS. Tsay SH. Chang FY. Lee SD. Triple therapy for the eradication of Helicobacter pylori and reduction of duodenal ulcer relapse: comparison of 1 week and 2 week regimens and recrudescence rates over 12 months. Journal of Gastroenterology and Hepatology 1995;10(3):300‐5. - PubMed
Chen 1996a {published data only}
-
- Chen S, Chen Z, Bei L. Omeprazole, clarithromycin and amoxicillin therapy for Helicobacter pylori infection [Chinese]. Zhonghua Nei Ke Za Zhi (National Medical Journal of China) 1996;35(12):799‐802. - PubMed
Chen 1996b {published data only}
-
- Chen SP, Lian B, Wen SH. Triple therapy with omeprazole, clarithromycin and amoxicillin for cure of Helicobacter pylori infection. Gut 1996;39 Suppl 3:A145. Abstract #823.
Ching 1997 {published data only}
-
- Ching CK, Chan YK, Ng WC. The combination of omeprazole, amoxycillin and clarithromycin eradicates Helicobacter Pylori in 95% of cases‐7 day equals 10‐days therapy. Gastroenterology 1997;112 Suppl 1:A87. - PubMed
Choi 2006 {published data only}
-
- Choi M, Oh JH, Kwon JH, Palk CN, Park JM, Choi YK, et al. Effect of CYP2C19 genotype on the eradication rate of Helicobacter pylori infection by pantoprazole based triple therapy in Koreans. Gut. 2006; Vol. UEGW Suppl:abstract MON‐G‐51.
Chun 2010 {published data only}
-
- Chun H, Hyun J, Park S, Keum B, Seo Y. The 10‐day sequential therapy for Helicobacter pylori eradication in Korea: Less effective than expected. International Journal of Medicine 2010;40 Suppl 1:85. Abstract #69.
Daghaghzadeh 2005 {published data only}
-
- Dagagzadeh H, Emami MH, Karimi S, Esmaeili A. One‐week versus two‐week furazolidone based quadruple therapy as the first‐line treatment for Helicobacter pylori Infection in Iran. Canadian Journal of Gastroenterology 2005;19(Suppl C):R.0224. - PubMed
Dammann 1997 {published data only}
-
- Dammann HG, Folsch UR, Hahn EG, et al. 7 vs 14 day treatment with pantoprazole, clarithromycin and metronidazole for cure of H. pylori infection in duodenal ulcer patients. Gut 1997;41 Suppl 1:A95. Abstract 09/349.
de Silva 2004 {published data only}
-
- Silva HA, Hewavisenthi J, Pathmeswaran A, Dassanayake AS, Navaratne NM, Peiris R, et al. Comparison of one week and two weeks of triple therapy for the eradication of Helicobacter pylori in a Sri Lankan population: a randomised, controlled study. Ceylon Medical Journal 2004;49(4):118‐22. - PubMed
Di Caro 2001a {published data only}
-
- Caro S, Zocco MA, Cremonini F, Bartolozzi F, Armuzi A, Carloni E, et al. Efficacy of 5, 7, and 10 days levofloxacin‐based dual therapy for H. pylori eradication. Gut 2001;49 Suppl 3:Abstract 2534.
Di Caro 2001b {published data only}
-
- Caro S, Zocco MA, Cremonini F, Armuzi A, Santarelli L, Candelli E, et al. Efficacy of 5,7 and 10 days of levofloxacin‐based dual therapy for H pylori eradication. Gut 2001;49 Suppl 2:A105. Abstract 15/31.
Di Caro 2001c {published data only}
-
- Caro S, Zocco MA, Cremonini F, Armuzzi A, Candelli M, Torre HS, et al. Lack of efficacy of a levofloxacin‐based dual therapy for H. Pylori eradication. Gastroenterology 2001;120(5 Suppl 1):A581. Abstract 2953.
Di Caro 2002b {published data only}
-
- Caro S, Zocco M, Cremonini F, Candelli M, Bartolozzi F, Armuzzi A, et al. Different schedule of levofloxacin based therapy in H. pylori infection. Journal of Gastroenterology and Hepatology 2002;17 Suppl:A234. Abstract P.B.020.
Di Mario 1999 {published data only}
-
- Mario F, Dai Bo N, Battaglia G, Pilotto A, Franceschi M, Marin R, et al. Are 6 days of a triple clarithromycin based therapy sufficient to obtain the cure ofH. pylori infection?. Gut 1999;45 Suppl 5:A271. Abstract P1023.
Di Mario 2002 {published data only}
-
- Mario F, Aragona 1 G, Dal Bo N, Ingegnoli A, Cavestro GM, Moussa AM, et al. Potential use of lactoferrin in a 7 days triple therapy for Helicobacter pylori eradication: preliminary results. European Helicobacter Study Group. XVth International Workshop; abstract book. 2002:A98. Abstract 15.30.
Di Mario 2003b {published data only}
-
- Mario F, Aragona G, Dal Bo N, Ingegnoli A, Cavestro GM, Moussa AM, et al. Use of lactoferrin for Helicobacter pylori eradication. Preliminary results. Journal of Clinical Gastroenterology 2003;36(5):396‐8. - PubMed
Flores 2010 {published data only}
-
- Flores HB, Salvana A, Ang ER, Estanislao NI, Velasquez ME, Ong J, et al. Duration of proton‐pump inhibitor‐based triple therapy for Helicobacter pylori eradication: A meta‐analysis. Gastroenterology 2010;138(5 Suppl 1):S340.
Ford 2003 {published data only}
-
- Ford A, Moayyedi P. How can the current strategies for Helicobacter pylori eradication therapy be improved?. Canadian Journal of Gastroenterology 2003;17 Suppl B:36B‐40B. - PubMed
Fuccio 2007 {published data only}
-
- Fuccio L, Minardi ME, Zagari RM, Grilli D, Magrini N, Bazzoli F. Meta‐analysis: duration of first‐line proton‐pump inhibitor based triple therapy for Helicobacter pylori eradication. Annals of Internal Medicine 2007;147(8):553‐62. - PubMed
Gené 2006 {published data only}
-
- Gené E, Calvet X, Azagra R, Gisbert JP. Seven or ten days? Cost‐effectiveness study on the duration of H. pylori treatment in primary care [Siete o diez dias? Estudio de coste‐efectividad sobre la duracion del tratamiento de la infeccion por H. pylori en atencion primaria]. Atencion Primaria 2006;38(10):555‐62. - PMC - PubMed
Giasi‐Gonzalez 2004 {published data only}
-
- Giasi‐Gonzalez E, Martínez Vázquez M, Garza‐Gonzalez E, et al. Randomized controlled trial: 7 vs. 14 days of a triple therapy of amoxicillin‐clarithromycin rabeprazole for the eradication of Helicobacter pylori and its correlation with the CYP2C19 genotype. Helicobacter 2004;9(5):574. Abstract 11.19.
Gisbert 2005b {published data only}
-
- Gisbert JP, Dominguez‐Munoz A, Dominguez‐Martin A, Gisbert JL, Marcos S, Pajares JM. Esomeprazole‐based therapy in Helicobacter pylori eradication: Any effect by increasing the dose of esomeprazole or prolonging the treatment?. Helicobacter 2005;10(5):530. Abstract 11.07. - PubMed
Greenberg 2011 {published data only}
Habu 1997 {published data only}
-
- Habu Y, Mizuno S, Hirano S, Kiyota K, Hayakumo T, Inokuchi H, et al. Randomized comparison of 1 week and 2 weeks of omeprazole, amoxicillin and clarithromycin treatment for the cure of Helicobacter pylori infection in gastric and duodenal ulcer patients. Gastroenterology 1997;112 Suppl:A136.
Hasan 2010 {published data only}
Herba 2009 {published data only}
-
- Herba K, Szilagyi A, Fallone C. Initial therapy for Helicobacter pylori eradication in a Canadian real life setting: An interim analysis comparing 7 and 14 day triple therapy regimens. Canadian Journal of Gastroenterology 2009;23:Abstract #95.
Iwasaki 1997 {published data only}
-
- Iwasaki A, Nakajima N. Ushiyama K, et al. Study of the usefulness of measurement of serum pepsinogen in eradication therapy of Helicobacter pylori. Gastroenterology 1997;112 Suppl 1:A159. Abstract #G0625.
Jung 2006 {published data only}
-
- Jung S, Lee S, Koo J, Yim H, Lee H, Choi J, et al. Effecacy of proton pump inhibitor‐based triple therapy for eradicating Helicobacter pylori in patients with chronic liver disease and peptic ulcer. Helicobacter 2006;11:414. Abstract 17.06.
Jung 2008 {published data only}
-
- Jung HS, Shim KN, Kang MJ, Jung JM, Ha CY, Na YJ. Comparison of the H. pylori eradication rate according to the duration of first‐line triple therapy: a meta‐analysis. Gastroenterology 2008;134(4 Suppl 1):P‐268. Abstract #T1647.
Kamberoglou 2001 {published data only}
-
- Kamberoglou D, Polymeros D, Sanidas I, Doulgeroglou V, Savva S, Patra E, et al. Comparison of 1‐week vs. 2‐ or 4‐week therapy regimens with ranitidine bismuth citrate plus two antibiotics for Helicobacter pylori eradication. Alimentary Pharmacology and Therapeutics 2001;15(9):1493‐7. - PubMed
Kang 2000b {published data only}
-
- Kang CD, Chun HJ, Hur BW, Jeen YT, Lee HS, Song CW, et al. Significance of pretreatment 13CO2 as predictor of success for H. pylori eradication. Gastroenterology 2000;118 Suppl 2:Abstract 4715.
Karatapanis 2000 {published data only}
-
- Karatapanis S, Georgopoulos S, Papakonstantinou L, Papamarkos D, Mentis A, Artikis V. Rabeprazole 7‐days vs rabeprazole 10‐days triple therapy in the eradication of H. pylori infection ‐ A randomized study. Gut 2000;47 Suppl 1:A107. Abstract 15/35.
Karatapanis 2005 {published data only}
-
- Karatapanis S, Georgopoulos S, Skorda L, Papantoniou N, Komnianides K, Lisgos P, et al. Rabeprazole 7‐days vs. rabeprazole 10‐days vs. rabeprazole 14 days triple therapy in the eradication of H. pylori infection‐ a randomized study. Gastroenterology 2005;128(4 Suppl 2):A‐428. Abstract T958.
Karatapanis 2007 {published data only}
-
- Karatapanis S, Georgopoulos S, Skorda L, Papantoniou N, Mathaios I, Lisgos P, et al. Triple regimen of variable duration (7 days vs 10 days vs 14 days), based on rabeprazole, in the eradication of Helicobacter pylori. A randomized study. Gut 2007;56 (Suppl 3):Abstract #Mon‐G‐59.
Katicic 1996 {published data only}
-
- Katicic M, Presecki V, Marusic M, Prskalo M, Ticak B, Sabaric B, et al. Eradication of H. pylori infection in peptic ulcers with four different drug regimens. Gut 1996;39 Suppl 3:A144. Abstract # 817.
Katicic 2006 {published data only}
-
- Katicic M, Ticak M, Prskalo M, Naumovski‐Mihalic S, Fillipec‐Kanizaj T, Skurla B, et al. Eradication of Helicobacter pylori infection with two triple‐therapy regimes of 7, 10, and 14 days; four years experience. Helicobacter 2006;11:386. Abstract # 11.05.
Khu 2010 {published data only}
-
- Khu JV, Lim LL. Helicobacter pylori eradication regimens: Is there a difference?. Journal of Pharmacy Practice and Research 2010;40(3):194‐8.
Kim 2001 {published data only}
-
- Kim YB, Chun HJ, JeenYT, Lee JW, Song CW. H. pylori eradication rate according to the duration of triple therapy in Korea: meta‐analysis evaluation of H. pylori trial. Gastroenterology 2001;120(5 Suppl 1):A‐583. Abstract#2965.
Knigge 1998 {published data only}
-
- Knigge, K, Kelly, C, Fennerty, MB, Peterson WK. A multicenter randomized trial with twice daily ranitidine bismuth‐citrate (R), metronidazole (M) and tetracycline (T) for 7 or 10 days for eradication of H‐pylori infection. Gastroenterology 1998;114(4 Suppl 2):Abstract G0736.
Krause 1997 {published data only}
-
- Krause R, Pruitt R, Lukasik N, Thomas J, Fennerty B. 10 vs 14 day triple therapy with lansoprazole (Prevacid), amoxicillin, and clarithromycin in the eradication of Helicobacter pylori (Hp). Gut 1997;41 Suppl:A101. Abstract 09/373.
Labenz 1993 {published data only}
-
- Labenz J, Gyenes E, Ruhl GH, Borsch G. Omeprazole plus amoxicillin: efficacy of various treatment regimens to eradicate Helicobacter pylori. American Journal of Gastroenterology 1993;88(4):491‐5. - PubMed
Lamouliatte 1998 {published data only}
-
- Lamouliatte H, Forestier S, Perie F. Lansoprazole (Lanso) 30 mg or 60 mg combined with two antibiotics (AMOX) and clarithromycin (Clari) to eradicate Helicobacter pylori (H. pylori). Gut 1998;43 Suppl 2:A80‐1. Abstract 10/275.
Lamouliatte 2000 {published data only}
-
- Lamouliatte H, Perie F, Joubert‐Collin M. Lansoprazole 30 mg or 60 mg combined with two antibiotics (amoxicillin and clarithromycin) to eradicate Helicobacter pylori in patients with duodenal ulcers [French]. Gastroenterologie Clinique et Biologique 2000;24(5):495‐500. - PubMed
Lanza 2002 {published data only}
-
- Lanza F, Kovacs T, Hahne W, Barth J, Sontag S. Rabeprazole‐based triple therapy is safe and well tolerated in Helicobacter pylori eradication: findings from a large US multicenter trial. American Journal of Gastroenterology 2002;97 Suppl 9:S24. Abstract 71.
Lee 1997 {published data only}
-
- Lee SW, Jin YT, Chun HJ, Um SH, Kim CD, Ryu HS, Hyun JH. The patients' compliance of anti‐Helicobacter pylori triple regimen with low dose clarithromycin in Korea. Gut 1997;41 (UEGW Suppl):Abstract P745.
Lee 1998 {published data only}
-
- Lee KH, Kim NY, Ko YH, et al. Triple therapy for eradication of H. pylori in patients with peptic ulcer [Korean]. Korean Journal of Gastroenterology 1998;31(5):605‐14.
Lee 2004b {published data only}
-
- Lee S, Keum B, Chung R, Lee H, Um S, Choi J, et al. Comparison of Helicobacter pylori eradication rate according to proton pump inhibitor‐based triple therapy: omeprazole, rabeprazole, esomeprazole and lansoprazole. Helicobacter 2004;9:579. Abstract 11.34. - PubMed
Lee 2006 {published data only}
-
- Lee EJ, Gham CW, Park TW, et al. The effect of Helicobacter pylori eradication on the improvement of the symptoms in patients with functional dyspepsia and peptic ulcer disease. [Korean]. Korean Journal of Medicine 2006;71(2):141‐8.
Liao 2002 {published data only}
-
- Liao CC, Lee CL, Lee HY, Wu CH, Chen TK, Tu TC, et al. Efficacy of different therapies for Helicobacter pylori eradication pylori eradication: A 5‐year experience in a single center. American Journal of Gastroenterology 2002;97 Suppl:S245. Abstract 748.
Lopez‐Roman 2011 {published data only}
-
- Lopez‐Roman O, Warrington E, Cruz‐Correa MR, Toro DH. 10‐Day and 14‐day sequential therapy vs. standard triple therapy for helicobacter pylori infection in a Puerto Rican treatment‐naive population: An interim analysis. Gastroenterology 2011;140(5 Suppl 1):S149.
Maconi 1998 {published data only}
-
- Maconi G, Imbesi V, Lazzaroni M, Bargiggia S, DiPidtro C, Morelli P, et al. One vs two week regimens of lansoprazole (L), amoxicillin (A), and clarithromycin (C) for duodenal ulcer (DU) healing and H. pylori (Hp) eradication. Digestion 1998;59 Suppl 3:419. Abstract ExhB3164.
Maconi 1999 {published data only}
-
- Maconi G, Imbesi V, Russo A, et al. Prolongation of a PPI‐based triple therapy from one to two weeks is of benefit to patients with high H. pylori density and current smokers. Gut 1999;45 Suppl V:A5. Abstract 05.03.
Makarenka 2004 {published data only}
-
- Makarenka AU, Pimanau SI. Results of eradication with classical triple and furazolidone‐based regimens in patients with high bacterial density of Helicobacter pylori. Helicobacter 2004;9(5):580. Abstract 11.37.
Makarenka 2005 {published data only}
-
- Makarenka AV, Pimanov SI. Eradication rate after randomized treatment in a population with high prevalence of Helicobacter pylori infection. Helicobacter. Helicobacter 2005;10:535. Abstract #11.22.
Matougui 2009 {published data only}
-
- Matougui N, Boudjella ML, Mouffok F, Bouhadef A, Guechi Z, Bouzid K, et al. H. pylori eradication by four triple therapies: Randomized study in double‐blind. Helicobacter 2009;14 (4):403.
Miwa 1998 {published data only}
-
- Miwa H, Ohkura R, Murai T, Nagahara A, Yamada T, Ogihara T, et al. Effectiveness of omeprazole‐amoxicillin‐clarithromycin (OAC) therapy for Helicobacter pylori infection in a Japanese population. Helicobacter 1998;3(2):132‐8. - PubMed
Moayyedi 1995 {published data only}
-
- Moayyedi P, Tompkins DS, Shanaghan K, Langworthy H, Axon ATR. Comparison of one and 2 weeks of lansoprazole, amoxycillin and clarithromycin in eradicating H.pylori. Gut 1995;37 Suppl 2:A7. Abstract W25.
Nishikawa 1999 {published data only}
-
- Nishikawa K, Sugiyama T, Ishizuka J, Kudo T, Komatsu Y, Katagiri M, et al. Eradication of Helicobacter pylori using 30 mg or 60 mg lansoprazole combined with amoxicillin and metronidazole: one and two weeks of a new triple therapy. Journal of Gastroenterology 1999;34 Suppl XI:72‐5. - PubMed
Ogura 2000 {published data only}
-
- Ogura K, Yoshida H, Maeda S, Yamaji Y, Mitsuno Y, Akanuma M, et al. Triple therapy with faropenem against drug‐resistant helicobacter pylori. Gastroenterology 2000;118 Suppl 2:Abstract #2679.
Ogura 2007 {published data only}
-
- Ogura K, Mitsuno Y, Maeda S, Hirata Y, Yanai A, Shibata W, et al. Efficacy and safety of faropenem in eradication therapy of Helicobacter pylori. Helicobacter 2007;12(6):618‐22. - PubMed
Oh 2006 {published data only}
-
- Oh JH, Choi M, Dong M, Park J, Kwon J, Pail C, et al. Effect of CYP2C19 genotype on the eradication rate of Helicobacter pylori infection by pantoprazole based triple therapy in Koreans. Gut 2006;55 (UEGW suppl):Abstract MON‐G‐51.
Oqura 2007 {published data only}
-
- Ogura K, Mitsuno Y, Maeda S, Hirata Y, Yanai A, Shibata W, et al. Efficacy and safety of faropenem in eradication therapy of Helicobacter pylori. Helicobacter 2007;12(6):618‐22. - PubMed
Paoluzi 2001 {published data only}
-
- Paoluzi P, Lacopini F, Consolazio A, et al. Two week PPI‐based triple therapy with amoxycillin and clarithromycin has a higher efficacy in Helicobacter pylori eradication. Gut 2001;49 Suppl 3:A 2545.
Park 2009b {published data only}
-
- Park SH, Chun HJ, Kim ES, Park SC, Jung ES, Lee SD, et al. The 10‐day sequential therapy for Helicobacter pylori eradication in Korea: Less effective than expected. Gastroenterology 2009;136(5 Suppl 1):A399‐40. Abstract#M1053.
Pimanov 2005 {published data only}
-
- Pimanov SI, Makarenka AV, Matveenko ME. Eradication and reinfection rates after randomized treatment in a population with high prevalence of Helicobacter pylori infection. Gut 2005;54 Suppl 7:A127. Abstract. MON‐G‐214.
Rodionoff 1990 {published data only}
-
- Rodionoff P, Hyland L, Ostapowicz N, et al. Triple therapy for Helicobacter pylori eradication: 1, 2 or 4 weeks?. Sydney, Australia: World Congress of Gastroenterology. August, 1990:Abstract PP938.
Rodríguez 2003 {published data only}
-
- Rodríguez W, Pareja Cruz A, Yushimito L, Ramírez Ramos A, Gilman RH, Watanabe Yamamoto J, et al. Omeprazole, amoxicillin and clarithromycin in the treatment of helicobacter pylori, in 7 and 10‐day regimens [Tratamiento del Helicobacter Pylori con Omeprazole, Amoxicilina y Clarithromicina en esquemas de 7 y 10 dias]. Revista Gastroenterología del Perú 2003;23(3):177‐83. - PubMed
Rodríguez Téllez 1999 {published data only}
-
- Rodríguez Téllez M, Valenzuela Barranco M, Caballero Plasencia A, Martín Ruiz J, López‐Andrade A, Carmona Soria I, et al. Morphometric estimation of acid output in duodenal ulcer associated with Helicobacter pylori infection. Revista Española de Enfermedades de Digestivas 1999;91(8):549‐58. - PubMed
Rogha 2009 {published data only}
-
- Rogha M, Pourmoghaddas Z, Rezaee M, Shirneshan K, Shahi Z. Azithromycin effect on helicobacter pylori eradication: double blind randomized clinical trial. Hepatogastroenterology 2009;56(91‐92):722‐4. - PubMed
Sacco 2008 {published data only}
-
- Sacco F, Spezzaferro M, Serio M, Amitrano M, Cerasa B, Grossi L, Marzio L. Effect of three different regimens with esomeprazole, amoxicillin, and moxifloxacin in the treatment of primary H. pylori Infection. Gastroenterology 2008;134(4 Suppl):A24. Abstract 140.
Salandin 1995 {published data only}
-
- Salandin S, Battaglia G, Dal Bo N, Lecis PE, Pilotto A, Ferrana M, et al. Clarithromycin for the cure of H.pylori infection: one or 2 weeks of treatment?. Gastroenterology 1995;108:A207.
Schwartz 2002 {published data only}
-
- Schwartz H, Monkemuller K, Perdomo C, Barth J, Stanton D. Helicobacter pylori eradication with rabeprazole‐based triple therapy: findings in 803 patients with antibiotic‐sensitive and resistant strains. American Journal of Gastroenterology 2002;97(9 Suppl):S21. Abstract 63.
Sharma 2002 {published data only}
-
- Sharma MP, Chaudhary A, Ahuja V. Three weeks triple therapy is better than two or one week therapy for eradication of Helicobacter pylori in peptic ulcer disease. Journal of Gastroenterology and Hepatology 2002;17 Suppl:A24. Abstract P.B.005.
Simsek 2000 {published data only}
-
- Simsek I. Reinfection rate of Helicobacter Pylori infection after one and two weeks triple. A 17‐month follow‐up study. Gut 2000;47 Suppl 3:A103. Abstract P296.
Utzon 1994 {published data only}
-
- Utzon P, Aabakken L, Sandstad O, Guldvog I, Skar V. Eradication of helicobacter pylori with 10 or 14 days of bismuth triple regimes. Gut 1994;35 Suppl IV:A150.
Uygun 1999 {published data only}
-
- Uygun A, Ates Y, Erdil A, Kadayifci A, Cetin C, Gulsen M, et al. Efficacy of omeprazole plus two antimicrobials for the eradication of Helicobacter pylori in a Turkish population. Clinical Therapeutics 1999;21(9):1539‐48. - PubMed
Vakil 2002a {published data only}
-
- Vakil N, Schwartz H, Lanza F, Nardi L, Hahne W, Barth J. A prospective, controlled, randomised trial of 3‐,7‐ and 10‐day rabeprazole based triple therapy for H. pylori eradication in the USA. Gastroenterology 2002;122(4 Suppl):A 65. Abstract 551.
Vakil 2002b {published data only}
-
- Vakil N, Schwarz H, Lanza F, Hahne W, Barth J. Seven‐day rabeprazole‐based triple therapy for H. pylori eradication is as effective as 10‐day omeprazole‐based triple therapy. European Helicobacter Study Group. XVth International Workshop 2002. Abstract book. 2002:A95. Abstract 15.19.
Vakil 2002c {published data only}
-
- Vakil N, Kovacs T, Ignatowitz W, Hahne W, Barth J. Presence or absence of peptic ulcer disease does not affect H. pylori eradication rates with rabeprazole‐based triple therapy. European Helicobacter Study Group. XVth International Workshop 2002. Abstract book. 2002:A95. Abstract 15.09.
Vakil 2002d {published data only}
-
- Vakil N, Kovacs T, Barth J. Antibiotic resistance accounts for only one third of treatment failures with triple therapy for H.pylori. Gut 2002;51 Suppl 3:A215. Abstract TUE‐G‐488.
Vakil 2002e {published data only}
-
- Vakil N, Schwartz H, Lanza F, Barth J. Safety and tolerability of rabeprazole‐based triple therapy for Helicobacter eradication in a large US trial. Gut 2002;51 Suppl 3:A110. Abstract MON‐G‐412.
Vakil 2002f {published data only}
-
- Vakil N, Jayanty V, Stanton D, Perdomo C, Hahne W, Barth J. Antibiotic sensitivity in a large US multicenter trial of rabeprazole‐based triple therapy for H. pylori eradication. Gastroenterology 2002;122(4 Suppl 1):A9. Abstract 63.
Vakil 2002g {published data only}
-
- Vakil N, Schwartz H, Perdomo C, Barth J, Sontag S. Racial demographics do not affect efficacy of rabeprazole‐based triple therapy for Helicobacter pylori eradication. American Journal of Gastroenterology 2002;97(9 Suppl):S17. Abstract 50.
Vakil 2002h {published data only}
-
- Vakil N, Stanton D, Hahne W, Barth J, Kovacs T. Consistent Helicobacter pylori eradication rates with rabeprazole‐based triple therapy across demographic subgroups. American Journal of Gastroenterology 2002;97(9 Suppl):S21. Abstract 62.
Vakil 2003a {published data only}
-
- Vakil N, Perdomo C, Barth J, et al. Clarithromycin resistance and H. pylori eradication rates in US racial groups with rabeprazole‐based triple therapy. Gastroenterology 2003;123(4 Suppl 1):Abstract M1495.
Vakil 2003b {published data only}
-
- Vakil N, Perdomo C, Barth J. Density of Helicobacter pylori (Hp) organisms does not affect eradication rates in peptic ulcer disease (PUD) and non‐peptic ulcer disease (NPUD). Gastroenterology 2003;123(4 Suppl 1):Abstract S1244.
Vakil 2003c {published data only}
-
- Vakil N, Perdomo C, Barth J. The effect of gastritis on Helicobacter pylori (Hp) eradication rates with triple therapy. Gastroenterology 2003;123(4 Suppl 1):Abstract 771.
Xiang 2007 {published data only}
-
- Xiang L. Wen FQ. Zuo WH. Tang Y. Different courses of esomeprazole‐based triple therapy for Helicobactor pylori infection in children. Zhongguo Dangdai Erke Zazhi. (Chinese Journal of Contemporary Pediatrics) 2007;9(3):205‐6. [http://en.cnki.com.cn/Article_en/CJFDTOTAL‐DDKZ200703005.htm] - PubMed
Zhou 2009 {published data only}
-
- Zhou L, Feng H, Lin S, Xue Y, Yang X. Analysis of related factors of H. pylori eradication therapy efficiency. Helicobacter 2009;14(4):333. Abstract W7.6.
Zullo 2004 {published data only}
-
- Zullo A, Scaccianoce G, Hassan C, et al. Helicobacter pylori eradication with 7‐day triple therapy, 10‐day regimen, and sequential therapy. Gut 2004;53 Suppl 6:A120. Abstract MON‐G‐236.
References to studies awaiting assessment
Chen 2013 {published data only}
-
- Chen W, Zhang GY, Zeng Y, Li Q, Xu MH, Liu T. Furazolidone‐based quadruple therapy as first‐line treatment for Helicobacter pylori infection [含呋喃唑酮四联一线方案初治幽门螺杆菌的临床观察]. World Chinese Journal of Digestology 2013;21(14):1366‐71.
Choi 2012 {published data only}
Fallone 2013 {published data only}
Konorev 2012 {published data only}
-
- Konorev MR. Use of the immunopotentiator N‐acetyl‐glucosamine‐N‐acetylmuramyl dipeptide during triple anti‐Helicobacter pylori therapy. Terapevticheskii Arkhiv 2012;84(12):66‐70. - PubMed
Lv 2013 {published data only}
-
- Lv N, Xie Y, Chen Y, Wang J, Liu W, Zhang Z, et al. Furazolidone‐based triple and quadruple eradication therapy for H. pylori infection. Journal of Gastroenterology and Hepatology. 2013; Vol. 28 (Suppl 3):1‐22. Abastract 00029.
Prasertpetmanee 2013 {published data only}
-
- Prasertpetmanee S, Mahachai V, Vilaichone R. Reliable efficacy of 14‐day high dose PPI triple therapy for helicobacter pylori eradication independent effect of CYP2C19 genotype and high prevalence of metronidazole resistance in Thailand. Helicobacter. 2012; Vol. 17:99.
-
- Prasertpetmanee S, Mahachai V, Vilaichone RK. High efficacy of 14‐day high dose PPI triple therapy for H. pylori eradication independent effect of CYP2C19 genotype in Thailand. Journal of Gastroenterology and Hepatology. 2012; Vol. 27:61.
-
- Prasertpetmanee S, Mahachai V, Vilaichone RK. Improved efficacy of PPI‐amoxicillin‐clarithromycin triple therapy for H. Pylori eradication in low clarithromycin resistance areas or for tailored therapy. Gastroenterology. 2013; Vol. 144 (5 Suppl 1):S332. - PubMed
-
- Prasertpetmanee S, Mahachai V, Vilaichone RK. Improved efficacy of proton pump inhibitor ‐ amoxicillin ‐ clarithromycin triple therapy for Helicobacter pylori eradication in low clarithromycin resistance areas or for tailored therapy. Helicobacter 2013;18(4):270‐3. - PubMed
-
- Prasertpetmanee S, Mahachai V, Vilaichone RK. Improved efficacy of proton pump inhibitor ‐ amoxicillin ‐ clarithromycin triple therapy for Helicobacter pylori eradication in low clarithromycin resistance areas or for tailored therapy. Helicobacter. 2013; Vol. 18(4):270‐273. - PubMed
Wang 2013 {published data only}
-
- Wang SJ, Wang WH, Chi YX, Teng GG. Concomitant therapy with four drugs is more effective than triple therapy for H. pylori eradication. Journal of Gastroenterology and Hepatology. 2013; Vol. 28 (Suppl 3):543. Abstract P1373.
Xu 2012 {published data only}
-
- Xu X, Sun Q, Liao J, Liang X, Zhang Q, Liu W, et al. Evaluation of bismuth‐clarithromycin‐containing quadruple therapy for initial Helicobacter pylori eradication. Chinese Journal of Gastroenterology 2012;17(1):5‐9.
Additional references
Ables 2007
-
- Ables AZ, Simon I, Melton ER. Update on Helicobacter pylori treatment. American Family Physician 2007;75(3):351‐8. - PubMed
Altman 2002
Asaka 2010
-
- Asaka M, Kato M, Takahashi S, Fukuda Y, Sugiyama T, Ota H, et al. Guidelines for the management of Helicobacter pylori infection in Japan: 2009 revised edition. Helicobacter 2010;15(1):1‐20. - PubMed
Baker 2008
-
- Baker DE. Pylera plus omeprazole: quadruple treatment for Helicobacter pylori. Reviews in Gastroenterological Disorders 2008;8(1):33‐41. - PubMed
Basu 2012
-
- Basu PP. Response to Yuan and Leontiadis (Letters to the editor). The American Journal of Gastroenterology 2012;107(7):1107. - PubMed
Begg 1994
-
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088‐101. - PubMed
Begg 1996
-
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 1996;276(8):637‐9. - PubMed
Bradburn 2007
-
- Bradburn MJ, Deeks JJ, Berlin JA, Russell Localio A. Much ado about nothing: a comparison of the performance of meta‐analytical methods with rare events. Statistics in Medicine 2007;26(1):53‐77. - PubMed
Broutet 2003
-
- Broutet N, Tchamgoué S, Pereira E, Lamouliatte H, Salamon R, Mégraud F. Risk factors for failure of Helicobacter pylori therapy‐‐ results of an individual data analysis of 2751 patients. Alimentary Pharmacology and Therapeutics 2003;17(1):99‐109. - PubMed
Buring 1999
-
- Buring SM, Winner LH, Hatton RC, Doering PL. Discontinuation rates of Helicobacter pylori treatment regimens: a meta‐analysis. Pharmacotherapy 1999;19(3):324‐32. - PubMed
Buzás 2006
Calvet 2008
-
- Calvet X, Villoria A, Vergara M. Could increasing the duration of triple therapy be a clinically useful strategy?. Annals of Internal Medicine 2008;148(8):624. - PubMed
Cates 2002
Chey 2007
-
- Chey WD, Wong BC, Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. American Journal of Gastroenterology 2007;102(8):1808‐25. - PubMed
Choi 2007
-
- Choi HS, Park DI, Hwang SJ, Park JS, Kim HJ, Cho YK, et al. Double‐dose, new‐generation proton pump inhibitors do not improve Helicobacter pylori eradication rate. Helicobacter 2007;12(6):638‐42. - PubMed
Clarke 2005
-
- Clarke MJ. Individual patient data meta‐analyses. Best Practice and Research. Clinical Obstetrics and Gynaecology 2005;19(1):47‐55. - PubMed
Cochrane 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Cockburn 1987
Cohen 1968
-
- Cohen J. Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychological Bulletin 1968;70:213‐20. - PubMed
Cook 1995
Counsell 1994
Dal Bo'N 1998
-
- Dal Bo' N, Mario F, Battaglia G, Buda A, Leandro G, Vianello F, et al. Low dose of clarithromycin in triple therapy for the eradication of Helicobacter pylori: one or two weeks?. Journal of Gastroenterology and Hepatology 1998;13(3):288‐93. - PubMed
De Francesco 2010
-
- Francesco V, Giorgio F, Hassan C, Manes G, Vannella L, Panella C, et al. Worldwide H. pylori antibiotic resistance: a systematic review. Journal of Gastrointestinal and Liver Diseases 2010;19(4):409‐14. - PubMed
de Martel 2006
-
- Martel C, Parsonnet J. Helicobacter pylori infection and gender: a meta‐analysis of population‐based prevalence surveys. Digestive Diseases and Sciences 2006;51(12):2292‐301. - PubMed
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. - PubMed
Di Mario 2006
-
- Mario F, Cavallaro LG, Scarpignato C. 'Rescue' therapies for the management of Helicobacter pylori infection. Digestive Diseases 2006;24(1‐2):113‐30. - PubMed
Dore 2000
-
- Dore MP, Leandro G, Realdi G, Sepulveda AR, Graham DY. Effect of pretreatment antibiotic resistance to metronidazole and clarithromycin on outcome of Helicobacter pylori therapy: a meta‐analytical approach. Digestive Diseases and Sciences 2000;45(1):68‐76. - PubMed
Egger 1997
Egger 1997a
Egger 1997b
Essa 2009
Fischbach 2002
-
- Fischbach LA, Goodman KJ, Feldman M, Aragaki C. Sources of variation of Helicobacter pylori treatment success in adults worldwide: a meta‐analysis. International Journal of Epidemiology 2002;31(1):128‐39. - PubMed
Fischbach 2004
-
- Fischbach LA, Zanten S, Dickason J. Meta‐analysis: the efficacy, adverse events, and adherence related to first‐line anti‐Helicobacter pylori quadruple therapies. Alimentary Pharmacology and Therapeutics 2004;20(10):1071‐82. - PubMed
Fischbach 2007
-
- Fischbach L, Evans EL. Meta‐analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple first‐line therapies for Helicobacter pylori. Alimentary Pharmacology and Therapeutics 2007;26(3):343‐57. - PubMed
Fock 2008
-
- Fock KM, Talley N, Moayyedi P, Hunt R, Azuma T, Sugano K, et al. Asia‐Pacific consensus guidelines on gastric cancer prevention. Journal of Gastroenterology and Hepatology 2008;23(3):351‐65. - PubMed
Fock 2009
-
- Fock KM, Katelaris P, Sugano K, Ang TL, Hunt R, Talley NJ, et al. Second Asia‐Pacific Consensus Guidelines for Helicobacter pylori infection. Journal of Gastroenterology and Hepatology 2009;24(10):1587‐600. - PubMed
Ford 2004
-
- Ford A, Delaney B, Forman D, Moayyedi P. Eradication therapy in Helicobacter pylori positive peptic ulcer disease: systematic review and economic analysis. American Journal of Gastroenterology 2004;99:1833‐55. - PubMed
Ford 2006
Ford 2008
Ford 2010a
-
- Ford AC, Axon AT. Epidemiology of Helicobacter pylori infection and public health implications. Helicobacter 2010;15 Suppl 1:1‐6. - PubMed
Ford 2010b
-
- Ford AC, Guyatt GH, Talley NJ, Moayyedi P. Errors in the conduct of systematic reviews of pharmacological interventions for irritable bowel syndrome. American Journal of Gastroenterology 2010;105(2):280‐8. - PubMed
Forman 2000
-
- Forman D, Bazzoli F, Bennett C, Broutet N, Chiba N, Deeks JJ, et al. Therapies for the eradication of Helicobacter pylori. Cochrane Database of Systematic Reviews 2000, Issue 3.
Forman 2009
-
- Forman D, Delaney B, Kuipers E, Malthaner R, Moayyedi P, Gardener E, et al. Cochrane Upper Gastrointestinal and Pancreatic Diseases Grouop. About The Cochrane Collaboration (Cochrane Review Groups (CRGs) 2009, issue 2. [Art. No.: UPPERGI]
Freston 1997
-
- Freston JW. What remaining questions regarding Helicobacter pylori and associated diseases should be addressed by future research? View from North America. Gastroenterology 1997;113(6 Suppl):S163‐6. - PubMed
Friedrich 2007
Fuccio 2008
-
- Fuccio L, Laterza L, Zagari RM, Cennamo V, Grilli D, Bazzoli F. Treatment of Helicobacter pylori infection. BMJ 2008;337(a1454):746‐50. - PubMed
Fuccio 2009
-
- Fuccio L, Eusebi LH, Bazzoli F. Was it really an "ugly" meta‐analysis?. American Journal of Gastroenterology 2009;104(11):2853. - PubMed
Furuta 2007
-
- Furuta T, Shirai N, Kodaira M, Sugimoto M, Nogaki A, Kuriyama S, et al. Pharmacogenomics‐based tailored versus standard therapeutic regimen for eradication of H. pylori. Clinical Pharmacology and Therapeutics 2007;81(4):521‐8. - PubMed
Gatta 2009
-
- Gatta L, Vakil N, Leandro G, Mario F, Vaira D. Sequential therapy or triple therapy for Helicobacter pylori infection: systematic review and meta‐analysis of randomized controlled trials in adults and children. American Journal of Gastroenterology 2009;104(12):3069‐79. - PubMed
Gatta 2013
Gene 2003a
-
- Gené E, Calvet X, Azagra R, Gisbert JP. Triple vs. quadruple therapy for treating Helicobacter pylori infection: a meta‐analysis. Alimentary Pharmacology and Therapeutics 2003;17(9):1137‐43. - PubMed
Gene 2003b
-
- Gené E, Calvet X, Azagra R, Gisbert JP. Triple vs quadruple therapy for treating Helicobacter pylori infection: an updated meta‐analysis. Alimentary Pharmacology and Therapeutics 2003;18(5):543‐4. - PubMed
Gisbert 1999
-
- Gisbert JP, Pajares JM, Valle J. Ranitidine bismuth citrate therapy regimens for treatment of Helicobacter pylori infection: a review. Helicobacter 1999;4(1):58‐66. - PubMed
Gisbert 2001a
-
- Gisbert JP, Marcos S, Gisbert JL, Pajares JM. Helicobacter pylori eradication therapy is more effective in peptic ulcer than in non‐ulcer dyspepsia. European Journal of Gastroenterology and Hepatology 2001;13(11):1303‐7. - PubMed
Gisbert 2001b
-
- Gisbert JP, Hermida C, Pajares JM. Are twelve days of omeprazole, amoxicillin and clarithromycin better than six days for treating H. pylori infection in peptic ulcer and in non‐ulcer dyspepsia?. Hepatogastroenterology 2001;48(41):1383‐8. - PubMed
Gisbert 2003
-
- Gisbert JP, Khorrami S, Calvet X, Gabriel R, Carballo F, Pajares JM. Meta‐analysis: proton pump inhibitors vs. H2‐receptor antagonists‐‐their efficacy with antibiotics in Helicobacter pylori eradication. Alimentary Pharmacology and Therapeutics 2003;18(8):757‐66. - PubMed
Gisbert 2005c
-
- Gisbert JP. Potent gastric acid inhibition in Helicobacter pylori eradication. Drugs 2005;65 Suppl 1:83‐96. - PubMed
Gisbert 2007
-
- Gisbert JP, Pajares R, Pajares JM. Evolution of Helicobacter pylori therapy from a meta‐analytical perspective. Helicobacter 2007;12 Suppl 2:50‐8. - PubMed
Gisbert 2011
-
- Gisbert JP, Calvet X. Review article: non‐bismuth quadruple (concomitant) therapy for eradication of Helicobater pylori. Alimentary Pharmacology and Therapeutics 2011;34(6):604‐17. - PubMed
Gisbert 2012
-
- Gisbert JP, Calvet X, Cosme A, Almela P, Feu F, Bory F, et al. Long‐term follow‐up of 1,000 patients cured ofHelicobacter pylori infection following an episode of peptic ulcer bleeding. American Journal of Gastroenterology 2012;107:1197‐1204. - PubMed
Glanville 2006
Glossary 2008
-
- Anonymous. Glossary. Evidence‐based Medicine 2008;13(4):128. [DOI: 10.1136/ebm.13.4.128] - DOI
Gluud 2006
-
- Gluud LL. Bias in clinical intervention research. American Journal of Epidemiology 2006;163(6):493‐501. - PubMed
Golder 2006
Gonzalez 2011
-
- Gonzalez CA, Figueiredo C, Lic CB, Ferreira RM, Pardo ML, Ruiz Liso JM, et al. Helicobacter pylori cagA and vacA genotypes as predictors of progression of gastric preneoplastic lesions: A long‐term follow‐up in a high‐risk Area in Spain. American Journal of Gastroenterology 2011;106:867‐74. - PubMed
Graham 2008
Graham 2010
-
- Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 2010;59(8):1143‐53. - PubMed
Harbord 2006
-
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. - PubMed
Harbord 2006
-
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. - PubMed
Haynes 2005
Higgins 2002
-
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. - PubMed
Higgins 2003
Higgins 2011
-
- PT Higgins JPT, Altman DG, AC Sterne JAC, Cochrane Statistical Methods Group and the Cochrane Bias Methods Group (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated September 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hirschl 1996
-
- Hirschl AM, Rotter ML. Amoxicillin for the treatment of Helicobacter pylori infection. Journal of Gastroenterology 1996;31 Suppl 9:44‐7. - PubMed
Hollis 1999
Howden 1998
-
- Howden CW, Hunt RH. Guidelines for the management of Helicobacter pylori infection. American Journal of Gastroenterology 1998;93:2330‐8. - PubMed
Huang 1999
-
- Huang J, Hunt RH. The importance of clarithromycin dose in the management of Helicobacter pylori infection: a meta‐analysis of triple therapies with a proton pump inhibitor, clarithromycin and amoxycillin or metronidazole. Alimentary Pharmacology and Therapeutics 1999;13(6):719‐29. - PubMed
Huang 2008
Hunt 1996
-
- Hunt RH. The role of Helicobacter pylori in pathogenesis: the spectrum of clinical outcomes. Scandinavian Journal of Gastroenterology. Supplement 1996;220:3‐9. - PubMed
Hunt 1998
-
- Hunt R, Thomson AB. Canadian Helicobacter pylori consensus conference. Canadian Association of Gastroenterology. Canadian Journal of Gastroenterology 1998;12(1):31‐41. - PubMed
Hunt 1999
-
- Hunt RH, Fallone CA, Thomson AB. Canadian Helicobacter pylori Consensus Conference update: infections in adults. Canadian Helicobacter Study Group. Canadian Journal of Gastroenterology 1999;13(3):213. - PubMed
Hunt 2004
-
- Hunt R, Fallone C, Veldhuyzan van Zanten S, Sherman P, Smaill F, Flook N, et al. CHSG 2004 participants. Canadian Helicobacter Study Group Consensus Conference: Update on the management of Helicobacter pylori‐‐an evidence‐based evaluation of six topics relevant to clinical outcomes in patients evaluated for H pylori infection. Canadian Journal of Gastroenterology 2004;18(9):547‐54. - PubMed
Hunt 2011
-
- Hunt RH, Xiao SD, Megraud F, Leon‐Barua R, Bazzoli F, Merwe S, et al. Helicobacter pylori in developing countries. World Gastroenterology Organisation Global Guideline. Journal of Gastrointestinal & Liver Diseases. 2011;20(3):299‐304. - PubMed
Hutton 2010
-
- Hutton JL. Misleading statistics: The problems surrounding number needed to treat and number needed to harm. Pharmaceutical Medicine 2010;24(3):145‐9.
Ioannidis 2004
-
- Ioannidis JP, Lau J. Completeness of safety reporting in randomized trials: an evaluation of 7 medical areas. JAMA 2001;285(4):437‐43. - PubMed
Ioannidis 2007
Jüni 2001
Katelaris 2002
-
- Katelaris PH, Forbes GM, Talley NJ, Crotty B. A randomized comparison of quadruple and triple therapies for Helicobacter pylori eradication: The QUADRATE Study. Gastroenterology 2002;123(6):1763‐9. - PubMed
Kato 2011
-
- Kato H, Kagami Y, Kodaira T, Oka S, Oki Y, Chihara D, et al. Nodal relapse after Helicobacter pylori eradication in a patient with primary localized gastric mucosa‐associated Lymphoid tissue lymphoma. American Journal of Gastroenterology 2011;106:549‐51. - PubMed
Kim 2001b
-
- Kim YB, Chun HJ, Jeen YT, Lee JW, Song CW, et al. H. pylori eradication rate according to the duration of triple therapy in Korea: meta‐analysis evaluation of H. pylori trial. Gastroenterology 2001;120(5 Suppl 1):A‐583. Abstract#2965.
Kudo 2012
-
- Kudo T, Fujinami H, Ando T, Nishikawa J, Ogawa K, Hosokawa A, et al. Comparison of lafutidine and rabeprazole in 7‐day second‐line amoxicillin‐ and metronidazole‐containing triple therapy for Helicobacter pylori: a pilot study. Helicobacter 2012;17(4):277‐81. - PubMed
Laine 2003
-
- Laine L, Hunt R, El‐Zimaity H, Nguyen B, Osato M, Spénard J. Bismuth‐based quadruple therapy using a single capsule of bismuth biskalcitrate, metronidazole, and tetracycline given with omeprazole versus omeprazole, amoxicillin, and clarithromycin for eradication of Helicobacter pylori in duodenal ulcer patients: a prospective, randomized, multicenter, North American trial. American Journal of Gastroenterology 2003;98(3):562‐7. - PubMed
Laine 2012
-
- Laine L, Jensen DM. Management of Patients With Ulcer Bleeding. American Journal of Gastroenterology 2012;107:345‐60. - PubMed
Lam 1998
-
- Lam SK, Talley NJ. Report of the 1997 Asia Pacific Consensus Conference on the management of Helicobacter pylori infection. Journal of Gastroenterology and Hepatology 1998;13(1):1‐12. - PubMed
Larsen 2012
Leung 2000
-
- Leung WK, Graham DY. Clarithromycin for Helicobacter pylori infection. Expert Opinion on Pharmacotherapy 2000;1(3):507‐14. - PubMed
Liou 2013
-
- Liou JM, Chen CC, Chen MJ, Chen CC, Chang CY, Fang YJ, et al. Sequential versus triple therapy for the first‐line treatment of Helicobacter pylori: a multicentre, open‐label, randomised trial. Lancet 2013;381(9862):205‐13. - PubMed
Louw 1998
-
- Louw JA, Rensburg CJ, Moola S, Kotze D, Marks IN. Helicobacter pylori eradication and ulcer healing with daily lansoprazole, plus 1 or 2 weeks co‐therapy with amoxycillin and clarithromycin. Alimentary Pharmacology and Therapeutics 1998;12(9):881‐5. - PubMed
Luther 2008
-
- Luther J, Schoenfeld P, Moayyedi P, Vakil N, George S, Chey W. Triple versus quadruple therapy as primary treatment for Helicobacter pylori infection: a meta‐analysis of efficacy and tolerability. American Journal of Gastroenterology 2008;103 Suppl 1:S397‐8. Abstract#1015.
Luther 2010
-
- Luther J, Higgins PD, Schoenfeld PS, Moayyedi P, Vakil N, Chey WD. Empiric quadruple vs. triple therapy for primary treatment of Helicobacter pylori infection: Systematic review and meta‐analysis of efficacy and tolerability. American Journal of Gastroenterology 2010;105(1):65‐73. - PubMed
Malfertheiner 2007
Malfertheiner 2012a
-
- Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, Bazzoli F, et al. Management of Helicobacter pylori infection‐‐the Maastricht IV/ Florence Consensus Report. Gut 2012;61(5):646‐64. - PubMed
Malfertheiner 2012b
-
- Malfertheiner P, Selgrad M, Bornschein J. Helicobacter pylori: clinical management. Current Opinion in Gastroenterology 2012;28(6):608‐14. - PubMed
Malfertheriner 2002
-
- Malfertheiner P, Mégraud F, O'Morain C, Hungin AP, Jones R, Axon A, et al. Current concepts in the management of Helicobacter pylori infection‐‐the Maastricht 2‐2000 Consensus Report. Alimentary Pharmacology and Therapeutics 2002;16(2):167‐80. - PubMed
McColm 1996
-
- McColm AA, McLaren A, Klinkert G, Francis MR, Connolly PC, Grinham CJ, et al. Ranitidine bismuth citrate: a novel anti‐ulcer agent with different physico‐chemical characteristics and improved biological activity to a bismuth citrate‐ranitidine admixture. Alimentary Pharmacology and Therapeutics 1996;10(3):241‐50. - PubMed
McNicholl 2012
-
- McNicholl AG, Linares PM, Nyssen OP, Calvet X, Gisbert JP. Meta‐analysis: esomeprazole or rabeprazole vs. first‐generation pump inhibitors in the treatment of Helicobacter pylori infection. Alimentary Pharmacology and Therapeutics 2012;36(5):414‐25. [DOI: 10.1111/j.1365-2036.2012.05211.x] - DOI - PubMed
Moayyedi 2000
Moayyedi 2002
Moayyedi 2003
-
- Moayyedi P, Deeks J, Talley NJ, Delaney B, Forman D. An update of the Cochrane systematic review of Helicobacter pylori eradication therapy in nonulcer dyspepsia: resolving the discrepancy between systematic reviews. American Journal of Gastroenterology 2003;98:2621‐6. - PubMed
Moayyedi 2004
-
- Moayyedi P. Meta‐analysis: Can we mix apples and oranges?. American Journal of Gastroenterology 2004;99(12):2297‐301. - PubMed
Moayyedi 2007
-
- Moayyedi P. The health economics of Helicobacter pylori infection. Best Practice and Research. Clinical Gastroenterology 2007;21(2):347‐61. - PubMed
Moayyedi 2009
-
- Moayyedi P, Malfertheiner P. Editorial: Sequential therapy for eradication of Helicobacter pylori: a new guiding light or a false dawn?. American Journal of Gastroenterology 2009;104(12):3081‐3. - PubMed
Montori 2001
Moore 2002
Mégraud 2005
-
- Mégraud F. Update on therapeutic options for Helicobacter pylori‐related diseases. Current Infectious Disease Reports 2005;7(2):115‐20. - PubMed
Nexium 2012
-
- AstraZeneca Pharmaceuticals LP. The label approved on 01/20/2012 (PDF) for NEXIUM, NDA no. 022101. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021153s040,0219... 01/20/2012 .
NIH 1994
-
- NIH Consensus Conference. Helicobacter pylori in peptic ulcer disease. NIH Consensus Development Panel on Helicobacter pylori in Peptic Ulcer Disease. JAMA 1994;272(1):65‐9. - PubMed
Pilkington 2005
-
- Pilkington K, Boshnakova A, Clarke M, Richardson J. "No language restrictions" in database searches: what does this really mean?. Journal of Alternative and Complementary Medicine 2005;11(1):205‐7. - PubMed
Pilotto 2000
-
- Pilotto A, Franceschi M, Rassu M, Leandro G, Bozzola L, Furlan F, Mario F. Incidence of secondary Helicobacter pylori resistance to antibiotics in treatment failures after 1‐week proton pump inhibitor‐based triple therapies: a prospective study. Digestive and Liver Disease 2000;32(8):667‐72. - PubMed
Ren 2010
-
- Ren Q, Ma B, Yang K, Yan X. Lafutidine‐based triple therapy for Helicobacter pylori eradication. Hepatogastroenterology 2010;57(102‐103):1074‐81. - PubMed
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rodgers 2007
Rokkas 2009
-
- Rokkas T, Sechopoulos P, Robotis I, Margantinis G, Pistiolas D. Cumulative H. pylori eradication rates in clinical practice by adopting first and second‐line regimens proposed by the Maastricht III consensus and a third‐line empirical regimen. American Journal of Gastroenterology 2009;104(1):21‐5. - PubMed
Sanchez‐Delgado 2011
-
- Sanchez‐Delgado J, Gene E, Suarez D, Garcia‐Iglesias P, Brullet E, Gallach M, et al. Has H. pylori prevalence in bleeding peptic ulcer been underestimated? A meta‐regression. American Journal of Gastroenterology 2011;106:398‐405. - PubMed
Savarino 2011
-
- Savarino E, Assandri L, Giannini EG, Savarino V. Small intestinal bacterial overgrowth and Helicobacter pylori: Can they be cause of thrombocytopenia in patients with chronic liver disease. American Journal of Gastroenterology 2011;106:1171‐2. - PubMed
Schmid 1999
-
- Schmid CH, Whiting G, Cory D, Ross SD, Chalmers TC. Omeprazole plus antibiotics in the eradication of Helicobacter pylori infection: a meta‐regression analysis of randomized, controlled trials. American Journal of Therapeutics 1999;6(1):25‐36. - PubMed
Senn 2009
Simmonds 2005
-
- Simmonds MC, Higgins JP, Stewart LA, Tierney JF, Clarke MJ, Thompson SG. Meta‐analysis of individual patient data from randomized trials: a review of methods used in practice. Clinical Trials 2005;2(3):209‐17. - PubMed
Spénard 2005
-
- Spénard J, Aumais C, Massicotte J, Brunet JS, Tremblay C, Grace M, Lefebvre M. Effects of food and formulation on the relative bioavailability of bismuth biskalcitrate, metronidazole, and tetracycline given for Helicobacter pylori eradication. British Journal of Clinical Pharmacology 2005;60(4):374‐7. - PMC - PubMed
StatsDirect 2012 [Computer program]
-
- StatsDirect Ltd. StatsDirect®. Version 2.7.8. Altrincham, Cheshire, UK: StatsDirect Ltd, 2012.
Sterne 2000
-
- Sterne JA, Egger M. Funnel plots for detecting bias in meta‐analysis: Guidelines on choice of axis. Journal of Clinical Epidemiology 2001;54:1046‐55. - PubMed
Sugimoto 2009
Sung 2009
-
- Sung JJ, Kuipers EJ, El‐Serag HB. Systematic review: the global incidence and prevalence of peptic ulcer disease. Alimentary Pharmacology and Therapeutics 2009;29(9):938‐46. - PubMed
Sweeting 2004
-
- Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Statistics in Medicine 2004;23:1351‐75. - PubMed
Talley 2005a
-
- Talley NJ, Vakil N. Practice Parameters Committee of the American College of Gastroenterology. Guidelines for the management of dyspepsia. American Journal of Gastroenterology 2005;100(10):2324‐37. - PubMed
Talley 2005b
-
- Talley NJ, Vakil NB, Moayyedi P. American Gastroenterological Association technical review on the evaluation of dyspepsia. Gastroenterology 2005;129:1756‐80. - PubMed
Taylor 1997
-
- Taylor JL, Zagari M, Murphy K, Freston JW. Pharmacoeconomic comparison of treatments for the eradication of Helicobacter pylori. Archives of Internal Medicine 1997;157(1):87‐97. - PubMed
Treiber 2000
-
- Treiber G. Treating H. pylori shorter than one week‐‐a real future perspective?. Zeitschrift fur Gastroenterologie 2000;38(9):807‐12. - PubMed
Tuner 2012
-
- Turner L, Shamseer L, Altman DG, Weeks L, Peters J, Kober T, et al. Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.MR000030.pub2] - DOI - PMC - PubMed
Uygun 2007
-
- Uygun A, Kadayifci A, Yesilova Z, Ates Y, Safali M, Ilgan S, et al. Poor efficacy of ranitidine bismuth citrate‐based triple therapies for Helicobacter pylori eradication. Indian Journal of Gastroenterology 2007;26(4):174‐7. - PubMed
Vakil 2009
-
- Vakil N. H. pylori treatment: new wine in old bottles?. American Journal of Gastroenterology 2009;104(1):26‐30. - PubMed
Vallve 2002
-
- Vallve M, Vergara M, Gisbert JP, Calvet X. Single vs. double dose of a proton pump inhibitor in triple therapy for Helicobacter pylori eradication: a meta‐analysis. Alimentary Pharmacology and Therapeutics 2002;16(6):1149‐56. - PubMed
Vergara 2003
-
- Vergara M, Vallve M, Gisbert JP, Calvet X. Meta‐analysis: comparative efficacy of different proton‐pump inhibitors in triple therapy for Helicobacter pylori eradication. Alimentary Pharmacology and Therapeutics 2003;18(6):647‐54. - PubMed
Vilaichone 2006
-
- Vilaichone RK, Mahachai V, Graham DY. Helicobacter pylori diagnosis and management. Gastroenterology Clinics of North America 2006;35(2):229‐47. - PubMed
Villoria 2008
-
- Villoria A, Garcia P, Calvet X, Gisbert JP, Vergara M. Meta‐analysis: high‐dose proton pump inhibitors vs. standard dose in triple therapy for Helicobacter pylori eradication. Alimentary Pharmacology and Therapeutics 2008;28(7):868‐77. - PubMed
Watson 2011
-
- Watson JB, Moss SF. Will H. pylori stagger under the weight of this LOAD? A novel but expensive eradication regimen. American Journal of Gastroenterology 2011;106(11):1976‐7. - PubMed
Wilczynski 2007
Wolle 2007
-
- Wolle K. Malfertheiner P. Treatment of Helicobacter pylori. Best Practice & Research. Clinical Gastroenterology 2007;21(2):315‐24. - PubMed
Wong 2006
Wood 2008
Yuan 2009
-
- Yuan Y, Hunt RH. Response to Fuccio et al Was it really an "ugly" meta‐analysis?. American Journal of Gastroenterology 2009; Vol. 104, issue 11:2853‐4. - PubMed
Yuan 2012
-
- Yuan Y, Leontiadis GI. Levofloxacin‐based three‐antibiotic regimen for H. pylori eradication (Letters to the editor). American Journal of Gastroenterology 2012;107(7):1106‐7. - PubMed
Zhao 2008
-
- Zhao F, Wang J, Yang Y, Wang X, Shi R, Xu Z, Huang Z, Zhang G. Effect of CYP2C19 genetic polymorphisms on the efficacy of proton pump inhibitor‐based triple therapy for Helicobacter pylori eradication: a meta‐analysis. Helicobacter 2008;13(6):532‐41. - PubMed
Zullo 2013
-
- Zullo A, Scaccianoce G, Francesco V, Ruggiero V, D'Ambrosio P, Castorani L, et al. Concomitant, sequential, and hybrid therapy for H. pylori eradication: A pilot study. Clinics and Research in Hepatology and Gastroenterology 2013;Jun 5. pii: S2210‐7401(13)00090‐9:[Epub ahead of print]. [DOI: 10.1016/j.clinre.2013.04.003] - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

