The influence of AKT isoforms on radiation sensitivity and DNA repair in colon cancer cell lines
- PMID: 24338765
- PMCID: PMC3980041
- DOI: 10.1007/s13277-013-1465-9
The influence of AKT isoforms on radiation sensitivity and DNA repair in colon cancer cell lines
Abstract
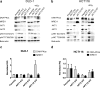
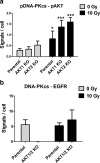
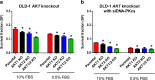
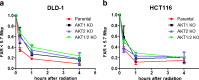
In response to ionizing radiation, several signaling cascades in the cell are activated to repair the DNA breaks, prevent apoptosis, and keep the cells proliferating. AKT is important for survival and proliferation and may also be an activating factor for DNA-PKcs and MRE11, which are essential proteins in the DNA repair process. AKT (PKB) is hyperactivated in several cancers and is associated with resistance to radiotherapy and chemotherapy. There are three AKT isoforms (AKT1, AKT2, and AKT3) with different expression patterns and functions in several cancer tumors. The role of AKT isoforms has been investigated in relation to radiation response and their effects on DNA repair proteins (DNA-PKcs and MRE11) in colon cancer cell lines. The knockout of AKT1 and/or AKT2 affected the radiation sensitivity, and a deficiency of both isoforms impaired the rejoining of radiation-induced DNA double strand breaks. Importantly, the active/phosphorylated forms of AKT and DNA-PKcs associate and exposure to ionizing radiation causes an increase in this interaction. Moreover, an increased expression of both DNA-PKcs and MRE11 was observed when AKT expression was ablated, yet only DNA-PKcs expression influenced AKT phosphorylation. Taken together, these results demonstrate a role for both AKT1 and AKT2 in radiotherapy response in colon cancer cells involving DNA repair capacity through the nonhomologous end joining pathway, thus suggesting that AKT in combination with DNA-PKcs inhibition may be used for radiotherapy sensitizing strategies in colon cancer.
Figures


References
-
- Krishnan S, Janjan N, Skibber J, Rodriguez-Bigas M, Wolff R, Das P, et al. Phase II study of capecitabine (Xeloda) and concomitant boost radiotherapy in patients with locally advanced rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2006;66(3):762–771. doi: 10.1016/j.ijrobp.2006.05.063. - DOI - PubMed
-
- Vaisman A, Varchenko M, Umar A, Kunkel TA, Risinger JI, Barrett JC, et al. The role of hMLH1, hMSH3, and hMSH6 defects in cisplatin and oxaliplatin resistance: correlation with replicative bypass of platinum-DNA adducts. Cancer Res. 1998;58(16):3579–3585. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

