Collagen as a double-edged sword in tumor progression
- PMID: 24338768
- PMCID: PMC3980040
- DOI: 10.1007/s13277-013-1511-7
Collagen as a double-edged sword in tumor progression
Abstract
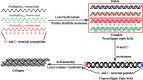
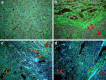
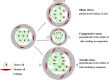
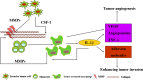
It has been recognized that cancer is not merely a disease of tumor cells, but a disease of imbalance, in which stromal cells and tumor microenvironment play crucial roles. Extracellular matrix (ECM) as the most abundant component in tumor microenvironment can regulate tumor cell behaviors and tissue tension homeostasis. Collagen constitutes the scaffold of tumor microenvironment and affects tumor microenvironment such that it regulates ECM remodeling by collagen degradation and re-deposition, and promotes tumor infiltration, angiogenesis, invasion and migration. While collagen was traditionally regarded as a passive barrier to resist tumor cells, it is now evident that collagen is also actively involved in promoting tumor progression. Collagen changes in tumor microenvironment release biomechanical signals, which are sensed by both tumor cells and stromal cells, trigger a cascade of biological events. In this work, we discuss how collagen can be a double-edged sword in tumor progression, both inhibiting and promoting tumor progression at different stages of cancer development.
Figures


References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Rosner M, Hengstschlager M. Targeting epigenetic readers in cancer. N Engl J Med. 2012;367:1764–5. - PubMed
-
- Dawson MA, Kouzarides T, Huntly BJ. Targeting epigenetic readers in cancer. N Engl J Med. 2012;367:647–57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

