ENaC activity is increased in isolated, split-open cortical collecting ducts from protein kinase Cα knockout mice
- PMID: 24338818
- PMCID: PMC3920049
- DOI: 10.1152/ajprenal.00519.2013
ENaC activity is increased in isolated, split-open cortical collecting ducts from protein kinase Cα knockout mice
Abstract
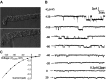
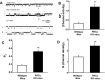
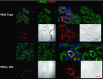
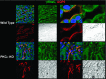
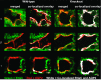
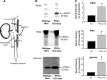
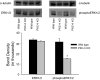
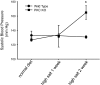
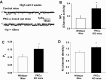
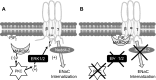
The epithelial Na channel (ENaC) is negatively regulated by protein kinase C (PKC) as shown using PKC activators in a cell culture model. To determine whether PKCα influences ENaC activity in vivo, we examined the regulation of ENaC in renal tubules from PKCα⁻/⁻ mice. Cortical collecting ducts were dissected and split open, and the exposed principal cells were subjected to cell-attached patch clamp. In the absence of PKCα, the open probability (P₀) of ENaC was increased three-fold vs. wild-type SV129 mice (0.52 ± 0.04 vs. 0.17 ± 0.02). The number of channels per patch was also increased. Using confocal microscopy, we observed an increase in membrane localization of α-, β-, and γ-subunits of ENaC in principal cells in the cortical collecting ducts of PKCα⁻/⁻ mice compared with wild-type mice. To confirm this increase, one kidney from each animal was perfused with biotin, and membrane protein was pulled down with streptavidin. The nonbiotinylated kidney was used to assess total protein. While total ENaC protein did not change in PKCα⁻/⁻ mice, membrane localization of all the ENaC subunits was increased. The increase in membrane ENaC could be explained by the observation that ERK1/2 phosphorylation was decreased in the knockout mice. These results imply a reduction in ENaC membrane accumulation and P₀ by PKCα in vivo. The PKC-mediated increase in ENaC activity was associated with an increase in blood pressure in knockout mice fed a high-salt diet.
Keywords: ENaC; hypertension; knockout mice; protein kinase Cα; renal tubules; single channels.
Figures


Comment in
-
PC and PKC: in vivo vs. in vitro.Am J Physiol Renal Physiol. 2014 Mar 1;306(5):F507-8. doi: 10.1152/ajprenal.00678.2013. Epub 2013 Dec 26. Am J Physiol Renal Physiol. 2014. PMID: 24370588 Free PMC article. No abstract available.
Similar articles
-
ENaC activity and expression is decreased in the lungs of protein kinase C-α knockout mice.Am J Physiol Lung Cell Mol Physiol. 2014 Sep 1;307(5):L374-85. doi: 10.1152/ajplung.00040.2014. Epub 2014 Jul 11. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 25015976 Free PMC article.
-
Adenylyl cyclase VI mediates vasopressin-stimulated ENaC activity.J Am Soc Nephrol. 2013 Feb;24(2):218-27. doi: 10.1681/ASN.2012050449. Epub 2012 Dec 20. J Am Soc Nephrol. 2013. PMID: 23264685 Free PMC article.
-
Mal protein stabilizes luminal membrane PLC-β3 and negatively regulates ENaC in mouse cortical collecting duct cells.Am J Physiol Renal Physiol. 2019 Oct 1;317(4):F986-F995. doi: 10.1152/ajprenal.00446.2018. Epub 2019 Jul 31. Am J Physiol Renal Physiol. 2019. PMID: 31364376 Free PMC article.
-
Role of Rho GDP dissociation inhibitor α in control of epithelial sodium channel (ENaC)-mediated sodium reabsorption.J Biol Chem. 2014 Oct 10;289(41):28651-9. doi: 10.1074/jbc.M114.558262. Epub 2014 Aug 27. J Biol Chem. 2014. PMID: 25164814 Free PMC article.
-
Regulation of ENaC in mice lacking renal insulin receptors in the collecting duct.FASEB J. 2013 Jul;27(7):2723-32. doi: 10.1096/fj.12-223792. Epub 2013 Apr 4. FASEB J. 2013. PMID: 23558339 Free PMC article.
Cited by
-
Alveolar nonselective channels are ASIC1a/α-ENaC channels and contribute to AFC.Am J Physiol Lung Cell Mol Physiol. 2017 Jun 1;312(6):L797-L811. doi: 10.1152/ajplung.00379.2016. Epub 2017 Mar 10. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 28283476 Free PMC article.
-
Lack of urea transporters, UT-A1 and UT-A3, increases nitric oxide accumulation to dampen medullary sodium reabsorption through ENaC.Am J Physiol Renal Physiol. 2019 Mar 1;316(3):F539-F549. doi: 10.1152/ajprenal.00166.2018. Epub 2018 Dec 12. Am J Physiol Renal Physiol. 2019. PMID: 30539654 Free PMC article.
-
Kidney-targeted inhibition of protein kinase C-α ameliorates nephrotoxic nephritis with restoration of mitochondrial dysfunction.Kidney Int. 2018 Aug;94(2):280-291. doi: 10.1016/j.kint.2018.01.032. Epub 2018 May 4. Kidney Int. 2018. PMID: 29731111 Free PMC article.
-
Knockout of mitochondrial voltage-dependent anion channel type 3 increases reactive oxygen species (ROS) levels and alters renal sodium transport.J Biol Chem. 2018 Feb 2;293(5):1666-1675. doi: 10.1074/jbc.M117.798645. Epub 2017 Nov 27. J Biol Chem. 2018. PMID: 29180450 Free PMC article.
-
Lovastatin-Induced Phosphatidylinositol-4-Phosphate 5-Kinase Diffusion from Microvilli Stimulates ROMK Channels.J Am Soc Nephrol. 2015 Jul;26(7):1576-87. doi: 10.1681/ASN.2013121326. Epub 2014 Oct 27. J Am Soc Nephrol. 2015. PMID: 25349201 Free PMC article.
References
-
- Alli AA, Gower WR., Jr The C type natriuretic peptide receptor tethers AHNAK1 at the plasma membrane to potentiate arachidonic acid-induced calcium mobilization. Am J Physiol Cell Physiol 297: C1157–C1167, 2009 - PubMed
-
- Alli AA, Gower WR., Jr Molecular approaches to examine the phosphorylation state of the C type natriuretic peptide receptor. J Cell Biochem 110: 985–994, 2010 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

