A concise radiosynthesis of the tau radiopharmaceutical, [(18) F]T807
- PMID: 24339014
- PMCID: PMC4114396
- DOI: 10.1002/jlcr.3098
A concise radiosynthesis of the tau radiopharmaceutical, [(18) F]T807
Abstract
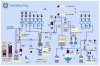
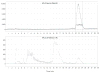
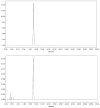
Fluorine-18 labeled 7-(6-fluoropyridin-3-yl)-5H-pyrido[4,3-b]indole ([(18) F]T807) is a potent and selective agent for imaging paired helical filaments of tau and is among the most promising PET radiopharmaceuticals for this target in early clinical trials. The present study reports a simplified one-step method for the synthesis of [(18) F]T807 that is broadly applicable for routine clinical production using a GE TRACERlab™ FXFN radiosynthesis module. Key facets of our optimized radiosynthesis include development and use of a more soluble protected precursor, tert-butyl 7-(6-nitropyridin-3-yl)-5H-pyrido[4,3-b]indole-5-carboxylate, as well as new HPLC separation conditions that enable a facile one-step synthesis. During the nucleophilic fluorinating reaction with potassium cryptand [(18) F]fluoride (K[(18) F]/K222 ) in DMSO at 130 °C over 10 min the precursor is concurrently deprotected. Formulated [(18) F]T807 was prepared in an uncorrected radiochemical yield of 14 ± 3%, with a specific activity of 216 ± 60 GBq/µmol (5837 ± 1621 mCi/µmol) at the end of synthesis (60 min; n = 3) and validated for human use. This methodology offers the advantage of faster synthesis in fewer steps, with simpler automation that we anticipate will facilitate widespread clinical use of [(18) F]T807.
Keywords: PET; T807; fluorine-18; tau.
Copyright © 2013 John Wiley & Sons, Ltd.
References
-
- Villemagne VL, Furumoto S, Fodero-Tavoletti M, Harada R, Mulligan RS, Kudo Y, Masters CL, Yanai K, Rowe CC, Okamura N. Future Neurology. 2012;7:409–421.
-
- Small GW, Kepe V, Ercoli LM, Siddarth P, Bookheimer SY, Miller KJ, Lavretsky H, Burggren AC, Cole GM, Vinters HV, Thompson PM, Huang SC, Satyamurthy N, Phelps ME, Barrio JR. New England J Med. 2006;355:2652–63. - PubMed
-
- Riss PJ, Brichard L, Ferrari V, Williamson DJ, Fryer TD, Hong YT, Baron JC, Aigbirhio FI. Med Chem Commun. 2013;4:852–855.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources