Childhood Arthritis and Rheumatology Research Alliance consensus treatment plans for new-onset polyarticular juvenile idiopathic arthritis
- PMID: 24339215
- PMCID: PMC4467832
- DOI: 10.1002/acr.22259
Childhood Arthritis and Rheumatology Research Alliance consensus treatment plans for new-onset polyarticular juvenile idiopathic arthritis
Abstract
Objective: There is no standardized approach to the initial treatment of polyarticular juvenile idiopathic arthritis (JIA) among pediatric rheumatologists. Understanding the comparative effectiveness of the diverse therapeutic options available will result in better health outcomes for polyarticular JIA. The Childhood Arthritis and Rheumatology Research Alliance (CARRA) developed consensus treatment plans (CTPs) for use in clinical practice to facilitate such studies.
Methods: A case-based survey was administered to CARRA members to identify the common treatment approaches for new-onset polyarticular JIA. Two face-to-face consensus conferences employed modified nominal group technique to identify treatment strategies, operational case definition, end points, and data elements to be collected. A core workgroup reviewed the relevant literature, refined plans, and developed medication dosing and monitoring recommendations.
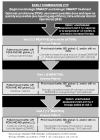
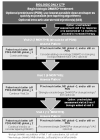
Results: The initial case-based survey identified significant variability among treatment approaches for new-onset polyarticular JIA. We developed 3 CTPs based on treatment strategies for the first 12 months of therapy, as well as case definitions and clinical and laboratory monitoring schedules. The CTPs include a step-up plan (nonbiologic disease-modifying antirheumatic drug [DMARD] followed by a biologic DMARD), an early combination plan (nonbiologic and biologic DMARD combined within a month of treatment initiation), and a biologic only plan. This approach was approved by 96% of the CARRA JIA Research Committee members attending the 2013 CARRA face-to-face meeting.
Conclusion: Three standardized CTPs were developed for new-onset polyarticular JIA. Coupled with data collection at defined intervals, use of these CTPs will enable the study of their comparative effectiveness in an observational setting to optimize initial management of polyarticular JIA.
Copyright © 2014 by the American College of Rheumatology.
Figures

References
-
- Karvonen M, Viik-Kajander M, Moltchanova E, Libman I, LaPorte R, Tuomilehto J. Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes Care. 2000 Oct;23(10):1516–1526. - PubMed
-
- Sacks JJ, Helmick CG, Luo YH, Ilowite NT, Bowyer S. Prevalence of and annual ambulatory health care visits for pediatric arthritis and other rheumatologic conditions in the United States in 2001–2004. Arthritis Rheum. 2007 Dec 15;57(8):1439–1445. - PubMed
-
- Wallace CA, Huang B, Bandeira M, Ravelli A, Giannini EH. Patterns of clinical remission in select categories of juvenile idiopathic arthritis. Arthritis Rheum. 2005 Nov;52(11):3554–3562. - PubMed
-
- Ringold S, Seidel KD, Koepsell TD, Wallace CA. Inactive disease in polyarticular juvenile idiopathic arthritis: current patterns and associations. Rheumatology (Oxford) 2009 Aug;48(8):972–977. - PubMed
-
- Wallace CA, Giannini EH, Huang B, Itert L, Ruperto N. American College of Rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2011 Jul;63(7):929–936. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

