Cyclosporine/ketoconazole reduces treatment costs for nephrotic syndrome
- PMID: 24339519
- PMCID: PMC3841509
- DOI: 10.4103/0971-4065.120338
Cyclosporine/ketoconazole reduces treatment costs for nephrotic syndrome
Abstract
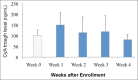
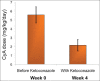
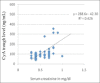
Cyclosporine A (CyA) is an effective agent for the treatment of glucocorticoid-dependent idiopathic nephrotic syndrome (GCDNS), but costs are prohibitive in resource-poor societies. The objectives of this study were to evaluate the efficacy and safety of reducing the dose of CyA by co-administering ketoconazole. A prospective study targeting children 2-18 years of age with GCDNS in remission with CyA monotherapy was conducted. CyA dose was reduced by 50% and ketoconazole was added at 25% of the recommended therapeutic dose, and the drug levels and therapeutic and adverse effects (AE) were monitored. Continued combined therapy after completion of the 4-week trial period was offered. Ten patients (median age 9.5 years, range 3.0-16.0 years) were enrolled in the study. At week 4, the CyA dose was 2.2 ± 0.7 mg/kg/day compared with 5.6 ± 0.9 mg/kg/day at enrolment (P < 0.0001). No AE were noted. All patients continued ketoconazole treatment for at least 3 months. CyA drug cost savings were 61%, and approximately 60% with ketoconazole cost included. The combination of an expensive immunosuppressive drug with a cheap metabolic inhibitor reduced the treatment costs by> 50% without increased adverse events or drug monitoring needs. This intervention demonstrates how access of patients with limited resources to needed drugs can be improved by interference with physiological drug elimination.
Keywords: Azoles; India; co-administration; cyclosporine; nephrotic syndrome; pediatric.
Conflict of interest statement
Figures
Similar articles
-
Effect of ketoconazole on cyclosporine dose in healthy dogs.Vet Surg. 1998 Jan-Feb;27(1):64-8. doi: 10.1111/j.1532-950x.1998.tb00099.x. Vet Surg. 1998. PMID: 9449179
-
Impact of the cyclosporine-ketoconazole interaction in children with steroid-dependent idiopathic nephrotic syndrome.Eur J Clin Pharmacol. 2006 Jan;62(1):3-8. doi: 10.1007/s00228-005-0064-0. Epub 2005 Dec 23. Eur J Clin Pharmacol. 2006. PMID: 16374637
-
Effect of cyclosporine therapy with low doses of corticosteroids on idiopathic nephrotic syndrome.Artif Organs. 2010 Mar;34(3):234-7. doi: 10.1111/j.1525-1594.2009.00838.x. Artif Organs. 2010. PMID: 20447050
-
[Cyclosporine and idiopathic extramembranous glomerulopathy. Hypothesis, facts and questions in 1992].Nephrologie. 1992;13(5):207-13. Nephrologie. 1992. PMID: 1470295 Review. French.
-
Long-term cyclosporin A therapy for severe idiopathic membranous nephropathy.Nephron. 1993;63(3):335-41. doi: 10.1159/000187219. Nephron. 1993. PMID: 8446273 Review.
Cited by
-
The Expression Pattern and Regulatory Mechanism of the G0/G1 Switch Gene 2 (G0S2) in the Pathogenesis and Treatment of AChR Myasthenia Gravis (MG).Mediators Inflamm. 2020 Sep 30;2020:4286047. doi: 10.1155/2020/4286047. eCollection 2020. Mediators Inflamm. 2020. PMID: 33061827 Free PMC article.
-
Management of focal segmental glomerulosclerosis in resource-limited regions.Pediatr Nephrol. 2024 Dec;39(12):3383-3386. doi: 10.1007/s00467-024-06430-5. Epub 2024 Jun 19. Pediatr Nephrol. 2024. PMID: 38896244 No abstract available.
-
Paediatric nephrology in under-resourced areas.Pediatr Nephrol. 2022 May;37(5):959-972. doi: 10.1007/s00467-021-05059-y. Epub 2021 Apr 10. Pediatr Nephrol. 2022. PMID: 33839937 Review.
References
-
- Bagga A, Mantan M. Nephrotic syndrome in children. Indian J Med Res. 2005;122:13–28. - PubMed
-
- van Husen M, Kemper MJ. New therapies in steroid-sensitive and steroid-resistant idiopathic nephrotic syndrome. Pediatr Nephrol. 2011;26:881–92. - PubMed
-
- Choudhry S, Bagga A, Hari P, Sharma S, Kalaivani M, Dinda A. Efficacy and safety of tacrolimus versus cyclosporine in children with steroid-resistant nephrotic syndrome: A randomized controlled trial. Am J Kidney Dis. 2009;53:760–9. - PubMed
-
- Tejani AT, Butt K, Trachtman H, Suthanthiran M, Rosenthal CJ, Khawar MR. Cyclosporine A induced remission of relapsing nephrotic syndrome in children. Kidney Int. 1988;33:729–34. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources