Macrophage migration inhibitory factor promotes proliferation and neuronal differentiation of neural stem/precursor cells through Wnt/β-catenin signal pathway
- PMID: 24339732
- PMCID: PMC3858584
- DOI: 10.7150/ijbs.7232
Macrophage migration inhibitory factor promotes proliferation and neuronal differentiation of neural stem/precursor cells through Wnt/β-catenin signal pathway
Abstract
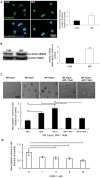
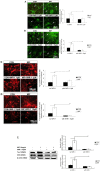
Macrophage migration inhibitory factor (MIF) is a highly conserved and evolutionarily ancient mediator with pleiotropic effects. Recent studies demonstrated that the receptors of MIF, including CD44, CXCR2, CXCR4 and CD74, are expressed in the neural stem/progenitor cells (NSPCs). The potential regulatory effect of MIF on NSPCs proliferation and neuronal differentiation, however, is largely unknown. Here, we investigated the effect of MIF on NSPC proliferation and neuronal differentiation, and further examined the signal pathway by which MIF transduced these signal effects in mouse NSPCs in vitro. The results showed that both Ki67-positive cells and neurosphere volumes were increased in a dose-dependent manner following MIF treatment. Furthermore, the expression of nuclear β-catenin was significantly stronger in MIF-stimulated groups than that in control groups. Conversely, administration of IWR-1, the inhibitor of Wnt/β-catenin pathway, significantly inhibited the proliferative effect of MIF on NSPCs. Immunostaining and Western blot further indicated that doublecortin (DCX) and Tuj 1, two neuronal markers, were evidently increased with MIF stimulation during NSPC differentiation, and there were more Tuj1-positive cells migrated out from neurospheres in MIF-stimulated groups than those in control groups. During NSPC differentiation, MIF increased the activity of β-galactosidase that responds to Wnt/β-catenin signaling. Wnt1 and β-catenin proteins were also up-regulated with MIF stimulation. Moreover, the expression of DCX and Tuj 1 was inhibited significantly by IWR-1. Taken together, the present study indicated that MIF enhances NSPC proliferation and promotes the neuronal differentiation, by activating Wnt/β-catenin signal pathway. The interaction between MIF and Wnt/β-catenin signal pathway may play an important role in modulating NSPC renewal and fate during brain development.
Keywords: MIF; NSPC; Wnt/β-catenin; neuronal differentiation; proliferation.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




References
-
- Massirer KB, Carromeu C, Griesi-Oliveira K, Muotri AR. Maintenance and differentiation of neural stem cells. Wiley Interdiscip Rev Syst Biol Med. 2011;3:107–14. - PubMed
-
- Moyse E, Segura S, Liard O, Mahaut S, Mechawar N. Microenvironmental determinants of adult neural stem cell proliferation and lineage commitment in the healthy and injured central nervous system. Curr Stem Cell Res Ther. 2008;3:163–84. - PubMed
-
- Bloom BR, Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966;153:80–2. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

