The epigenomics of embryonic stem cell differentiation
- PMID: 24339734
- PMCID: PMC3858586
- DOI: 10.7150/ijbs.7998
The epigenomics of embryonic stem cell differentiation
Abstract
Embryonic stem cells (ESCs) possess an open and highly dynamic chromatin landscape, which underlies their plasticity and ultimately maintains ESC pluripotency. The ESC epigenome must not only maintain the transcription of pluripotency-associated genes but must also, through gene priming, facilitate rapid and cell type-specific activation of developmental genes upon lineage commitment. Trans-generational inheritance ensures that the ESC chromatin state is stably transmitted from one generation to the next; yet at the same time, epigenetic marks are highly dynamic, reversible and responsive to extracellular cues. Once committed to differentiation, the ESC epigenome is remodeled and resolves into a more compact chromatin state. A thorough understanding of the role of chromatin modifiers in ESC fate and differentiation will be important if they are to be used for therapeutic purposes. Recent technical advances, particularly in next-generation sequencing technologies, have provided a genome-scale view of epigenetic marks and chromatin modifiers. More affordable and faster sequencing platforms have led to a comprehensive characterization of the ESC epigenome and epigenomes of differentiated cell types. In this review, we summarize and discuss the recent progress that has highlighted the central role of histone modifications, histone variants, DNA methylation and chromatin modifiers in ESC pluripotency and ESC fate. We provide a detailed and comprehensive discussion of genome-wide studies that are pertinent to our understanding of mammalian development.
Keywords: Epigenomics; chromatin; differentiation; embryonic stem cells.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

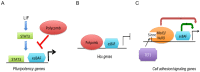

Similar articles
-
Histone variants as emerging regulators of embryonic stem cell identity.Epigenetics. 2015;10(7):563-73. doi: 10.1080/15592294.2015.1053682. Epigenetics. 2015. PMID: 26114724 Free PMC article. Review.
-
Returning to the stem state: epigenetics of recapitulating pre-differentiation chromatin structure.Bioessays. 2010 Sep;32(9):791-9. doi: 10.1002/bies.201000033. Bioessays. 2010. PMID: 20652894 Review.
-
Embryonic stem cell and induced pluripotent stem cell: an epigenetic perspective.Cell Res. 2013 Jan;23(1):49-69. doi: 10.1038/cr.2012.175. Epub 2012 Dec 18. Cell Res. 2013. PMID: 23247625 Free PMC article. Review.
-
Stem cells and reprogramming: breaking the epigenetic barrier?Trends Pharmacol Sci. 2011 Jul;32(7):394-401. doi: 10.1016/j.tips.2011.03.002. Epub 2011 May 27. Trends Pharmacol Sci. 2011. PMID: 21621281 Free PMC article. Review.
-
Concise review: epigenetic mechanisms contribute to pluripotency and cell lineage determination of embryonic stem cells.Stem Cells. 2007 Jan;25(1):2-9. doi: 10.1634/stemcells.2006-0383. Epub 2006 Oct 5. Stem Cells. 2007. PMID: 17023513 Review.
Cited by
-
The gene repressor complex NuRD interacts with the histone variant H3.3 at promoters of active genes.Genome Res. 2018 Nov;28(11):1646-1655. doi: 10.1101/gr.236224.118. Epub 2018 Sep 25. Genome Res. 2018. PMID: 30254051 Free PMC article.
-
DNA methylation study of fetus genome through a genome-wide analysis.BMC Med Genomics. 2014 Apr 15;7:18. doi: 10.1186/1755-8794-7-18. BMC Med Genomics. 2014. PMID: 24731722 Free PMC article.
-
Simultaneous epigenetic perturbation and genome imaging reveal distinct roles of H3K9me3 in chromatin architecture and transcription.Genome Biol. 2020 Dec 8;21(1):296. doi: 10.1186/s13059-020-02201-1. Genome Biol. 2020. PMID: 33292531 Free PMC article.
-
Dissecting the calcium-induced differentiation of human primary keratinocytes stem cells by integrative and structural network analyses.PLoS Comput Biol. 2015 May 6;11(5):e1004256. doi: 10.1371/journal.pcbi.1004256. eCollection 2015 May. PLoS Comput Biol. 2015. PMID: 25946651 Free PMC article.
-
Histone H4 acetylation and the epigenetic reader Brd4 are critical regulators of pluripotency in embryonic stem cells.BMC Genomics. 2016 Feb 4;17:95. doi: 10.1186/s12864-016-2414-y. BMC Genomics. 2016. PMID: 26847871 Free PMC article.
References
-
- Bradley A, Evans M, Kaufman MH, Robertson E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 1984;309:255–6. - PubMed
-
- Lanner F, Rossant J. The role of FGF/Erk signaling in pluripotent cells. Development. 2010;137:3351–60. - PubMed
-
- Li Z, Chen YG. Functions of BMP signaling in embryonic stem cell fate determination. Exp Cell Res. 2013;319:113–9. - PubMed
-
- Loebel DA, Watson CM, De Young RA, Tam PP. Lineage choice and differentiation in mouse embryos and embryonic stem cells. Dev Biol. 2003;264:1–14. - PubMed
-
- Okita K, Yamanaka S. Intracellular signaling pathways regulating pluripotency of embryonic stem cells. Curr Stem Cell Res Ther. 2006;1:103–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

