Alternative lengthening of telomeres: recurrent cytogenetic aberrations and chromosome stability under extreme telomere dysfunction
- PMID: 24339742
- PMCID: PMC3858894
- DOI: 10.1593/neo.131574
Alternative lengthening of telomeres: recurrent cytogenetic aberrations and chromosome stability under extreme telomere dysfunction
Abstract
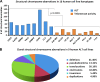
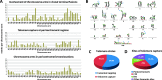
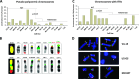
Human tumors using the alternative lengthening of telomeres (ALT) exert high rates of telomere dysfunction. Numerical chromosomal aberrations are very frequent, and structural rearrangements are widely scattered among the genome. This challenging context allows the study of telomere dysfunction-driven chromosomal instability in neoplasia (CIN) in a massive scale. We used molecular cytogenetics to achieve detailed karyotyping in 10 human ALT neoplastic cell lines. We identified 518 clonal recombinant chromosomes affected by 649 structural rearrangements. While all human chromosomes were involved in random or clonal, terminal, or pericentromeric rearrangements and were capable to undergo telomere healing at broken ends, a differential recombinatorial propensity of specific genomic regions was noted. We show that ALT cells undergo epigenetic modifications rendering polycentric chromosomes functionally monocentric, and because of increased terminal recombinogenicity, they generate clonal recombinant chromosomes with interstitial telomeric repeats. Losses of chromosomes 13, X, and 22, gains of 2, 3, 5, and 20, and translocation/deletion events involving several common chromosomal fragile sites (CFSs) were recurrent. Long-term reconstitution of telomerase activity in ALT cells reduced significantly the rates of random ongoing telomeric and pericentromeric CIN. However, the contribution of CFS in overall CIN remained unaffected, suggesting that in ALT cells whole-genome replication stress is not suppressed by telomerase activation. Our results provide novel insights into ALT-driven CIN, unveiling in parallel specific genomic sites that may harbor genes critical for ALT cancerous cell growth.
Figures

Similar articles
-
Pericentromeric instability and spontaneous emergence of human neoacrocentric and minute chromosomes in the alternative pathway of telomere lengthening.Cancer Res. 2008 Oct 1;68(19):8146-55. doi: 10.1158/0008-5472.CAN-08-0945. Cancer Res. 2008. PMID: 18829574
-
Resolving Roadblocks to Telomere Replication.Methods Mol Biol. 2019;1999:31-57. doi: 10.1007/978-1-4939-9500-4_2. Methods Mol Biol. 2019. PMID: 31127568 Review.
-
Frequency of chromosome healing and interstitial telomeres in 40 cases of constitutional abnormalities.Cytogenet Genome Res. 2009;125(3):176-85. doi: 10.1159/000230002. Epub 2009 Sep 4. Cytogenet Genome Res. 2009. PMID: 19738378
-
Functional Loss of ATRX and TERC Activates Alternative Lengthening of Telomeres (ALT) in LAPC4 Prostate Cancer Cells.Mol Cancer Res. 2019 Dec;17(12):2480-2491. doi: 10.1158/1541-7786.MCR-19-0654. Epub 2019 Oct 14. Mol Cancer Res. 2019. PMID: 31611308 Free PMC article.
-
Telomeres: Implications for Cancer Development.Int J Mol Sci. 2018 Jan 19;19(1):294. doi: 10.3390/ijms19010294. Int J Mol Sci. 2018. PMID: 29351238 Free PMC article. Review.
Cited by
-
Single-molecule analysis of subtelomeres and telomeres in Alternative Lengthening of Telomeres (ALT) cells.BMC Genomics. 2020 Jul 15;21(1):485. doi: 10.1186/s12864-020-06901-7. BMC Genomics. 2020. PMID: 32669102 Free PMC article.
-
ALT: A Multi-Faceted Phenomenon.Genes (Basel). 2020 Jan 27;11(2):133. doi: 10.3390/genes11020133. Genes (Basel). 2020. PMID: 32012790 Free PMC article. Review.
-
Molecular mechanisms of activity and derepression of alternative lengthening of telomeres.Nat Struct Mol Biol. 2015 Nov;22(11):875-80. doi: 10.1038/nsmb.3106. Epub 2015 Nov 4. Nat Struct Mol Biol. 2015. PMID: 26581522 Review.
-
SMARCAL1: Expanding the spectrum of genes associated with alternative lengthening of telomeres.Neuro Oncol. 2023 Sep 5;25(9):1576-1577. doi: 10.1093/neuonc/noad084. Neuro Oncol. 2023. PMID: 37163741 Free PMC article. No abstract available.
-
The Molecular Mechanisms and Therapeutic Prospects of Alternative Lengthening of Telomeres (ALT).Cancers (Basel). 2023 Mar 23;15(7):1945. doi: 10.3390/cancers15071945. Cancers (Basel). 2023. PMID: 37046606 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
