The TFPI-2 derived peptide EDC34 improves outcome of gram-negative sepsis
- PMID: 24339780
- PMCID: PMC3855554
- DOI: 10.1371/journal.ppat.1003803
The TFPI-2 derived peptide EDC34 improves outcome of gram-negative sepsis
Abstract
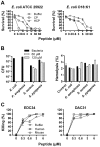
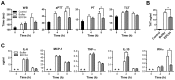
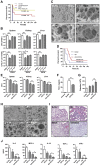
Sepsis is characterized by a dysregulated host-pathogen response, leading to high cytokine levels, excessive coagulation and failure to eradicate invasive bacteria. Novel therapeutic strategies that address crucial pathogenetic steps during infection are urgently needed. Here, we describe novel bioactive roles and therapeutic anti-infective potential of the peptide EDC34, derived from the C-terminus of tissue factor pathway inhibitor-2 (TFPI-2). This peptide exerted direct bactericidal effects and boosted activation of the classical complement pathway including formation of antimicrobial C3a, but inhibited bacteria-induced activation of the contact system. Correspondingly, in mouse models of severe Escherichia coli and Pseudomonas aeruginosa infection, treatment with EDC34 reduced bacterial levels and lung damage. In combination with the antibiotic ceftazidime, the peptide significantly prolonged survival and reduced mortality in mice. The peptide's boosting effect on bacterial clearance paired with its inhibiting effect on excessive coagulation makes it a promising therapeutic candidate for invasive Gram-negative infections.
Conflict of interest statement
I have read the journal's policy and have the following conflicts. The authors M. Malmsten and A. Schmidtchen are founders and own shares in XImmune AB, a company developing anti-inflammatory peptides for human therapy. This does not alter our adherence to all PLoS Pathogens policies on sharing data and materials.
Figures



Similar articles
-
Vertebrate TFPI-2 C-terminal peptides exert therapeutic applications against Gram-negative infections.BMC Microbiol. 2016 Jun 27;16(1):129. doi: 10.1186/s12866-016-0750-3. BMC Microbiol. 2016. PMID: 27349742 Free PMC article.
-
TFPI-2 Protects Against Gram-Negative Bacterial Infection.Front Immunol. 2018 Sep 11;9:2072. doi: 10.3389/fimmu.2018.02072. eCollection 2018. Front Immunol. 2018. PMID: 30254643 Free PMC article.
-
Antibacterial Properties of Tebipenem Pivoxil Tablet, a New Oral Carbapenem Preparation against a Variety of Pathogenic Bacteria in Vitro and in Vivo.Molecules. 2016 Jan 6;21(1):62. doi: 10.3390/molecules21010062. Molecules. 2016. PMID: 26751436 Free PMC article.
-
Evidence for antibiotic-mediated endotoxin release as a contributing factor to lethality in experimental gram-negative sepsis.Scand J Infect Dis Suppl. 1996;101:3-8. Scand J Infect Dis Suppl. 1996. PMID: 9060044 Review.
-
Pseudomonas aeruginosa Biofilms: Host Response and Clinical Implications in Lung Infections.Am J Respir Cell Mol Biol. 2018 Apr;58(4):428-439. doi: 10.1165/rcmb.2017-0321TR. Am J Respir Cell Mol Biol. 2018. PMID: 29372812 Free PMC article. Review.
Cited by
-
Structural basis for endotoxin neutralisation and anti-inflammatory activity of thrombin-derived C-terminal peptides.Nat Commun. 2018 Jul 17;9(1):2762. doi: 10.1038/s41467-018-05242-0. Nat Commun. 2018. PMID: 30018388 Free PMC article.
-
An ecoimmunological approach to study evolutionary and ancient links between coagulation, complement and Innate immunity.Virulence. 2018 Dec 31;9(1):724-737. doi: 10.1080/21505594.2018.1441589. Virulence. 2018. PMID: 29473457 Free PMC article.
-
Feasibility and Safety of Local Treatment with Recombinant Human Tissue Factor Pathway Inhibitor in a Rat Model of Streptococcus pneumoniae Pneumonia.PLoS One. 2015 May 18;10(5):e0127261. doi: 10.1371/journal.pone.0127261. eCollection 2015. PLoS One. 2015. PMID: 25992779 Free PMC article.
-
Antibiotic adjuvants: diverse strategies for controlling drug-resistant pathogens.Chem Biol Drug Des. 2015 Jan;85(1):56-78. doi: 10.1111/cbdd.12478. Chem Biol Drug Des. 2015. PMID: 25393203 Free PMC article.
-
Pseudomonas aeruginosa elastase cleaves a C-terminal peptide from human thrombin that inhibits host inflammatory responses.Nat Commun. 2016 May 16;7:11567. doi: 10.1038/ncomms11567. Nat Commun. 2016. PMID: 27181065 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

