Inhibition Studies on Enzymes Involved in Isoprenoid Biosynthesis: Focus on Two Potential Drug Targets: DXR and IDI-2 Enzymes
- PMID: 24339799
- PMCID: PMC3856697
- DOI: 10.2174/157340811796575317
Inhibition Studies on Enzymes Involved in Isoprenoid Biosynthesis: Focus on Two Potential Drug Targets: DXR and IDI-2 Enzymes
Abstract
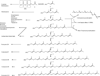
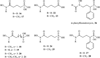
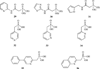
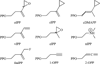
Isoprenoid compounds constitute an immensely diverse group of acyclic, monocyclic and polycyclic compounds that play important roles in all living organisms. Despite the diversity of their structures, this plethora of natural products arises from only two 5-carbon precursors, isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). This review will discuss the enzymes in the mevalonate (MVA) and methylerythritol phosphate (MEP) biosynthetic pathways leading to IPP and DMAPP with a particular focus on MEP synthase (DXR) and IPP isomerase (IDI), which are potential targets for the development of antibiotic compounds. DXR is the second enzyme in the MEP pathway and the only one for which inhibitors with antimicrobial activity at pharmaceutically relevant concentrations are known. All of the published DXR inhibitors are fosmidomycin analogues, except for a few bisphosphonates with moderate inhibitory activity. These far, there are no other candidates that target DXR. IDI was first identified and characterised over 40 years ago (IDI-1) and a second convergently evolved isoform (IDI-2) was discovered in 2001. IDI-1 is a metalloprotein found in Eukarya and many species of Bacteria. Its mechanism has been extensively studied. In contrast, IDI-2 requires reduced flavin mononucleotide as a cofactor. The mechanism of action for IDI-2 is less well defined. This review will describe how lead inhibitors are being improved by structure-based drug design and enzymatic assays against DXR to lead to new drug families and how mechanistic probes are being used to address questions about the mechanisms of the isomerases.
Keywords: DXR; IDI; MEP; MVA; isomerase; isopentenyl; isoprenoid; mevalonate; reductoisomerase.
Figures

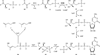





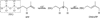



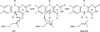
References
-
- Sacchettini JC, Poulter CD. Creating isoprenoid diversity. Science. 1997;277:1788–1789. - PubMed
-
- Cane DE. In: Comprehensive Natural Products Chemistry. Barton D, Nakanishi K, editors. Oxford: Elsevier; 1999.
-
- Wang KC, Ohnuma S. Isoprenyl diphosphate synthases. Biochim. Biophys. Acta. 2000;1529:33–48. - PubMed
-
- Bloch K. The biological synthesis of cholesterol. Science. 1965;150:19–28. - PubMed
-
- Matsumi R, Atomi H, Driessen AJM, van der Oost J. Isoprenoid biosynthesis in Archaea--biochemical and evolutionary implications. Res. Microbiol. 2011;162:39–52. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
