Loss of progesterone receptor-mediated actions induce preterm cellular and structural remodeling of the cervix and premature birth
- PMID: 24339918
- PMCID: PMC3858221
- DOI: 10.1371/journal.pone.0081340
Loss of progesterone receptor-mediated actions induce preterm cellular and structural remodeling of the cervix and premature birth
Abstract
A decline in serum progesterone or antagonism of progesterone receptor function results in preterm labor and birth. Whether characteristics of premature remodeling of the cervix after antiprogestins or ovariectomy are similar to that at term was the focus of the present study. Groups of pregnant rats were treated with vehicle, a progesterone receptor antagonist (onapristone or mifepristone), or ovariectomized on day 17 postbreeding. As expected, controls given vehicle delivered at term while rats delivered preterm after progesterone receptor antagonist treatment or ovariectomy. Similar to the cervix before term, the preterm cervix of progesterone receptor antagonist-treated rats was characterized by reduced cell nuclei density, decreased collagen content and structure, as well as a greater presence of macrophages per unit area. Thus, loss of nuclear progesterone receptor-mediated actions promoted structural remodeling of the cervix, increased census of resident macrophages, and preterm birth much like that found in the cervix at term. In contrast to the progesterone receptor antagonist-induced advance in characteristics associated with remodeling, ovariectomy-induced loss of systemic progesterone did not affect hypertrophy, extracellular collagen, or macrophage numbers in the cervix. Thus, the structure and macrophage census in the cervix appear sufficient for premature ripening and birth to occur well before term. With progesterone receptors predominantly localized on cells other than macrophages, the findings suggest that interactions between cells may facilitate the loss of progesterone receptor-mediated actions as part of a final common mechanism that remodels the cervix in certain etiologies of preterm and with parturition at term.
Conflict of interest statement
Figures
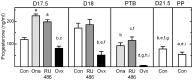

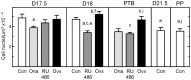
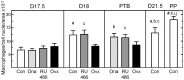
Similar articles
-
Progesterone Receptor-Mediated Actions Regulate Remodeling of the Cervix in Preparation for Preterm Parturition.Reprod Sci. 2016 Nov;23(11):1473-1483. doi: 10.1177/1933719116650756. Epub 2016 May 27. Reprod Sci. 2016. PMID: 27233754 Free PMC article.
-
Density of Stromal Cells and Macrophages Associated With Collagen Remodeling in the Human Cervix in Preterm and Term Birth.Reprod Sci. 2016 May;23(5):595-603. doi: 10.1177/1933719115616497. Epub 2015 Nov 25. Reprod Sci. 2016. PMID: 26608218 Free PMC article.
-
Progesterone regulation of cervix ripening in preterm and term birth.bioRxiv [Preprint]. 2025 Feb 1:2025.01.31.636012. doi: 10.1101/2025.01.31.636012. bioRxiv. 2025. PMID: 39974958 Free PMC article. Preprint.
-
Contributions to the dynamics of cervix remodeling prior to term and preterm birth.Biol Reprod. 2017 Jan 1;96(1):13-23. doi: 10.1095/biolreprod.116.142844. Biol Reprod. 2017. PMID: 28395330 Free PMC article. Review.
-
Immunobiology of Cervix Ripening.Front Immunol. 2020 Jan 24;10:3156. doi: 10.3389/fimmu.2019.03156. eCollection 2019. Front Immunol. 2020. PMID: 32038651 Free PMC article. Review.
Cited by
-
Cervix Stromal Cells and the Progesterone Receptor A Isoform Mediate Effects of Progesterone for Prepartum Remodeling.Reprod Sci. 2019 May;26(5):690-696. doi: 10.1177/1933719118820462. Epub 2019 Jan 17. Reprod Sci. 2019. PMID: 30654718 Free PMC article.
-
Vaginal progesterone, but not 17α-hydroxyprogesterone caproate, has antiinflammatory effects at the murine maternal-fetal interface.Am J Obstet Gynecol. 2015 Dec;213(6):846.e1-846.e19. doi: 10.1016/j.ajog.2015.08.010. Epub 2015 Aug 8. Am J Obstet Gynecol. 2015. PMID: 26264823 Free PMC article.
-
Vaginal progesterone to prevent preterm birth in pregnant women with a sonographic short cervix: clinical and public health implications.Am J Obstet Gynecol. 2016 Feb;214(2):235-242. doi: 10.1016/j.ajog.2015.09.102. Epub 2015 Oct 9. Am J Obstet Gynecol. 2016. PMID: 26450404 Free PMC article.
-
Progesterone alters human cervical epithelial and stromal cell transition and migration: Implications in cervical remodeling during pregnancy and parturition.Mol Cell Endocrinol. 2021 Jun 1;529:111276. doi: 10.1016/j.mce.2021.111276. Epub 2021 Apr 3. Mol Cell Endocrinol. 2021. PMID: 33823217 Free PMC article.
-
Immune Regulation in Pregnancy: A Matter of Perspective?Obstet Gynecol Clin North Am. 2016 Dec;43(4):679-698. doi: 10.1016/j.ogc.2016.07.004. Epub 2016 Oct 14. Obstet Gynecol Clin North Am. 2016. PMID: 27816154 Free PMC article. Review.
References
-
- Mackler AM, Iezza G, Akin MR, McMillan P, Yellon SM (1999) Macrophage trafficking in the uterus and cervix precedes parturition in the mouse. Biol Reprod 61: 879–883. - PubMed
-
- Kirby LS, Kirby MA, Warren JW, Tran LT, Yellon SM (2005) Increased innervation and ripening of the prepartum murine cervix. J Soc Gynecol Investig 12: 578–585. - PubMed
-
- Chwalisz K (1994) The use of progesterone antagonists for cervical ripening and as an adjunct to labour and delivery1. Hum Reprod 9 Suppl 1131–161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

